 |
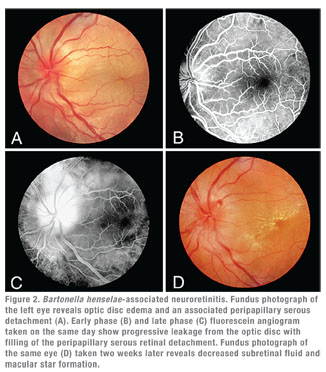 |
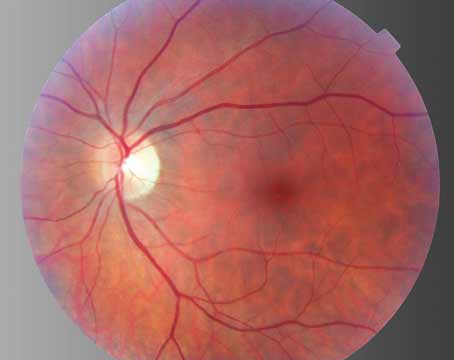
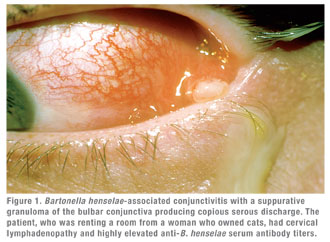
Clinically, cat scratch disease (CSD) is the most common presentation of B. henselae infection. Five to 10 percent of patients with CSD develop ocular involvement, most commonly either Parinaud’s Oculoglandular Syndrome (POGS; See Figure 1), which affects 5 percent, or neuroretinitis (See Figure 2), which occurs in 1 to 2 percent of symptomatic patients.10,11 POGS is characterized by tender regional lymphadenopathy of the preauricular, submandibular, or cervical lymph nodes associated with infection of the conjunctiva, eyelid or adjacent skin surface. Usual complaints include unilateral eye redness, foreign body sensation and epiphora. Discharge may be serous or purulent (See Figure 1) and can be copious in some patients.12 Neuroretinitis as described by Theodor Leber and later by Donald Gass is defined as acute unilateral visual loss associated with an exudative optic neuritis with transudation into the macula forming a partial or complete macular star (See Figure 2).13 Optic disc edema typically precedes the formation of a macular star by two to four weeks.14 Associated findings in patients with neuroretinitis may include peripapillary serous retinal detachment, focal retinitis or choroiditis, multifocal retinitis or choroiditis, and vasculitis—which may be occlusive.3,10 Bilateral Bartonella-associated neuroretinitis has been reported, but is rare and should prompt consideration of malignant hypertension, pseudotumor cerebri or bilateral diabetic papillopathy.15 One patient with Bartonella-associated neuroretinitis was reported to have developed acutely elevated intraocular pressure, so-called Inflammatory Ocular Hypertension Syndrome, accompanied by neovascularization of the retina.16,17
Bartonella-Associated Uveitis
Bartonella-associated uveitis can be unilateral or bilateral; anterior, intermediate, or posterior; granulomatous or nongranulomatous; and with differing degrees of posterior segment signs. Though there may be a history of contact with cats, such an exposure history is not required to make the diagnosis.12 The first published description of Bartonella-associated uveitis was a case of nongranulomatous anterior uveitis without posterior segment findings.18 Since then, a number of posterior segment complications have been described. Most common, perhaps, are retinal infiltrates, which are typically superficial, round, and 100 to 300 µm in size. They are usually multiple and often occur in the setting of neuroretinitis.13,21,22 These spots usually clear with no residual scarring in two to three weeks.13,19,20 In addition to multifocal retinitis, there have also been descriptions of focal choroiditis in patients with CSD, and areas of both retinitis and choroiditis may be associated with an adjacent or overlying exudative retinal detachment and/or retinal vascular occlusion.13,19,20 Bartonella infections have also been associated with diffuse choroiditis, focal retinal vasculitis and peripapillary angiomatosis.20,22-24 Most patients with Bartonella-associated neuroretinitis or retinochoroiditis show some degree of vitreous or anterior segment inflammation. Intraocular inflammation can also occur in the absence of focal retinochoroiditis or optic disc involvement, however.25,26
 |
Over the past two decades, Bartonella species have been identified as an emerging and under recognized cause of systemic disease among homeless and immunocompromised individuals.27,28 In immunocompromised and HIV-infected patients, Bartonell-associated retinochoroiditis has been reported to be larger than the more typical multifocal retinitis described above, and can be associated with neurosensory detachment and/or vascular changes.27,29,30 This clinical presentation is similar to retinitis caused by other infections and, thus, is not pathognomonic for Bartonella-associated disease.29 In some immunocompromised patients, Bartonella organisms can establish persistent infection in vascular endothelial cells, producing a vasoproliferative response.30,31 Histologically, these bacillary angiomatosis lesions are similar to the vascular lesions seen in the Kaposi’s sarcoma—and have been reported in association with infections from B. henselae, B. quintana and B. bacilliformis, the etiologic agent in verruca peruana.31 This vasoproliferative response can perhaps be best seen with fluorescin or indocyanine green angiography. Though there are reported cases of ocular CSD getting better with no specific treatment, lesions can enlarge and disease can progress in immunocompromised patients if diagnosis and treatment are delayed.29,32 Treatment is generally recommended, therefore, in the setting of immunocompromise.
Evidence on HLA-B27
Many Gram-negative bacteria (Klebsiella, Salmonella, Proteus, Yersinia, Shigella and Campylobacter) have been implicated in the development of HLA-B27-associated diseases such as uveitis, reactive arthritis and ankylosing spondylitis.33 There is also emerging evidence of a potential association between Bartonella intraocular infections/uveitis and HLA-B27 antigen expression. Michel Drancourt and associates described a 40-year-old HLA-B27 positive woman with a 10-year history of poorly controlled nongranulomatous uveitis whose inflammation was controlled only after a Bartonella diagnosis was made and appropriate antibiotic therapy was instituted.34 In an analysis done by Frank Kerkhoff and Aniki Rothova, six of 19 patients with positive enzyme immunoassays for B. henselae were also HLA-B27 positive. This number was significantly higher than the rate of HLA-B27 positivity in a control group of 25 patients with uveitis and no serologic evidence for B. henselae infection.33
Moreover, HLA-B27 positive patients may be at risk for more severe ocular Bartonella infections. Five of the six patients with both serologic evidence of Bartonella infection and HLA-B27 antigen expression described in this study had what Drs. Kerkhoff and Rothova characterized as severe posterior segment involvement. Four of these patients had a history of previous uveitis restricted to the anterior segment, and only later developed a more severe posterior uveitis when they converted to having B. henselae-positive serology.33 It is currently unknown how such bacterial colonization or infection with B. henselae (and other Gram-negative bacteria) triggers or worsens HLA-associated disease, but there are theories of localized changes related to the interactions between the bacteria and immune cells in HLA-B27-positive patients.33
Diagnosis
The diagnosis of CSD has historically been made clinically, but the development of biomolecular laboratory techniques such as sensitive and specific serologic tests and polymerase chain reaction analyses are now used to aid in the identification of B. henselae infection in most patients. In the United States, there are two types of serologic tests currently used for the diagnosis of CSD. In 1992, the Centers for Disease Control and Prevention began offering an indirect fluorescent assay specific for antibodies directed against B. henselae. This test has a sensitivity and specificity above 90 percent, but it does not differentiate between IgM and IgG antibodies.8 The other commercially available serologic test is an enzyme-linked immunoassay for both IgG and IgM anti-B. henselae antibodies. These ELISA tests are currently available at multiple laboratories and have variable sensitivity and specificity.35 With both IFA and ELISA, IgM tests are more accurate in the first eight weeks of CSD symptoms, while the IgG tests can remain accurate for longer periods of time or can be found in patients without any evidence of ocular or systemic CSD.25,35 Most of these tests have cross-reactivity to B. quintana, and some have cross-reactivity to other Bartonella species as well. PCR-based techniques have also been created. These tests are very sensitive and can differentiate between Bartonella species, but they are not yet widely available and thus far have mostly been used in research institutions. Culture of Bartonella species is possible, but it is difficult and infrequently used for diagnostic or therapeutic purposes.
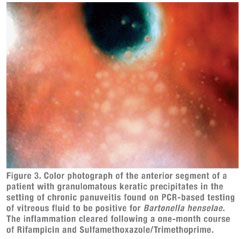
Though serologic markers of Bartonella infection were linked to ocular CSD in the late 1990s, the prevalence and full spectrum of Bartonella-associated uveitic disease was difficult to ascertain because of limited numbers of patients in the original case reports.11,19 These issues were partly addressed by Michel Drancourt and colleagues, who evaluated a population of 1,520 cases of previously idiopathic uveitis.12 Using both serology (IFA) confirmed with Western blot analysis and PCR analysis of intraocular fluids, the investigators found that 21 of 78 fastidious bacteria responsible for the uveitis were Bartonella species (B. henselae 9, B. quintana 7, B. grahamii 4, other Bartonella species1). Eighteen of these 21 patients were identified serologically, and three were identified using intraocular specimens. Of the 21 patients, 10 had bilateral disease. The uveitis was granulomatous in 11 cases (See Figure 3), nongranulomatous in 10 cases, posterior in 11 cases, anterior in eight cases and intermediate in two cases.
When and how to test for Bartonella infection in patients with uveitis remains the subject of debate. Most agree that serologic testing for B. henselae exposure is indicated for patients presenting with well-recognized complications of CSD, including signs of POGS, neuroretinitis or focal retinitis or choroiditis of the type described in patients with known B. henselae infection. While a single positive IgG supports the diagnosis of bartonellosis in patients with characteristic clinical features, either a positive IgM or a fourfold change in IgG titers provides the most compelling evidence for association in patients with an atypical presentation, particularly since 3 to 5 percent of the general population has positive B. henselae titers.25
It is less clear when to perform testing for exposure to other Bartonella species. Because uveitis caused by Bartonella species other than B. henselae appears to be uncommon, and, since the reported clinical presentations in such patients have been fairly non-descript, such testing is typically reserved for those patients with systemic signs or symptoms suggesting infection by a specific Bartonella species. Where available, broad PCR-based screening of aqueous or vitreous for bacterial and viral DNA, including Bartonella species, may be considered in patients with chronic uveitis refractory to treatment. We do not typically test for B. henselae infection in patients with HLA-B27-associated anterior uveitis, or for HLA-B27 positivity in patients with ocular complications of CSD, both because the degree of correlation appears to be limited and because a positive result would not alter management.
Treatment
Doxycycline, 100 mg orally b.i.d., is usually prescribed for two to four weeks in immunocompetent patients and up to four months in the immunocompromised.4 Treatment can also be continued for longer periods in immunocompromised patients to help prevent recurrence of ocular disease.36 Doxycycline augments the free radicals used by polymorphonuclear cells to kill phagocitized cells, which are suppressed by Bartonella infections.19 Doxycycline is preferred to erythromycin for its better intraocular and central nervous system penetration, but doxycycline should not be given to young children, in whom tooth discoloration is a possibility. For severe disease or in patients with central nervous system involvement, these same antibiotics can also be given intravenously, or together with rifampin, 300 mg orally b.i.d.36 Patients with severe optic nerve or macular inflammation may benefit from corticosteroids in addition to antimicrobial treatment.22
Bartonella species are well-recognized ocular pathogens. B. henselae is most frequently implicated, with POGS and neuroretinitis being the most common manifestations. Associated findings in patients with neuroretinitis include peripapillary serous retinal detachment, focal retinitis, focal choroiditis, vasculitis and vascular occlusion. More recent studies suggest that infection by diverse Bartonella species may be associated with uveitis and that the inflammation may be granulomatous or non-granulomatous; anterior, intermediate, posterior, or diffuse; and with or without neuroretinitis or peripapillary serous retinal detachment.
Both serologic and intraocular fluid tests are now available to diagnose Bartonella-associated uveitis, but when and how to use these tests remains a subject of debate. Short-term treatment with oral doxycycline is usually adequate to control the ocular manifestations in immunocompetent patients, but longer treatment with multiple agents may be necessary in patients with HIV/AIDS.
Dr. Chappell is a resident in ophthalmology at California Pacific Medical Center in San Francisco. Dr. Jumper is the Retina Service chief at CPMC and in private practice at the West Coast Retina Medical Group. Dr. McDonald is an attending at CPMC and in private practice at the West Coast Retina Medical Group. Dr. Cunningham is director of the Uveitis Service at CPMC, an adjunct clinical professor of ophthalmology at Stanford University School of Medicine, Stanford, Calif., and in private practice at West Coast Retina Medical Group.
Address correspondence to Dr. Cunningham at West Coast Retina Medical Group, Lobby 2, Ste. 130, 185 Berry St., San Francisco, CA 94107. Phone: (415) 972-4600; fax: (415) 975-0999; e-mail:
emmett_cunningham@yahoo.com. This work was supported in part by the Pacific Vision Foundation and by the San Francisco Retina Foundation.
(Figures 1 and 2 are reproduced with permission from International Ophthalmology Clinics; see reference 37. Figure 3 and clinical history provided courtesy of Professor Bahram Bodaghi, Paris, France.)
1. Golnik KC, Marotto ME, Fanous MM, et al. Ophthalmic manifestations of Rochalimaea species. Am J Ophthalmol 1994;118:145–151.
2. Dehio C. Molecular and Cellular Basis of Bartonella Pathogenesis. Annu Rev Microbiol 2004;58:365–90.
3. Roe HR, Jumper JM, Fu AD, et al. Ocular Bartonella Infections. International Ophthalmology Clinics 2008;48:93-105.
4. Rolain JM, Brouqui P, Koehler JE, et al. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother 2004;48: 1921–1933.
5. O’Halloran HS, Draud K, Minix M, et al. Leber’s neuroretinitis in a patient with serologic evidence of Bartonella elizabethae. Retina 1998;18:276–278.
6. Borboli S, Afshari NA, Watkins L, et al. Presumed oculoglandular syndrome from Bartonella quintana. Ocul Immunol Inflamm 2007;15:41–43.
7. Kerkhoff FT, Bergmans AM, van Der Zee A, et al. Demonstration of Bartonella grahamii DNA in ocular fluids of a patient with neuroretinitis. J Clin Microbiol 1999;37:4034–4038.
8. Dalton MJ, Robinson LE, Cooper J, et al. Use of Bartonella antigens for serologic diagnosis of cat-scratch disease at a national referral center. Arch Intern Med 1995; 155:1670–1676
9. Zangwill KM, Hamilton DH, Perkins BA, et al. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 1993;329:8-13.
10. Carithers HA. Cat-scratch disease. An overview based on a study of 1,200 patients. Am J Dis Child 1985;139:1124–1133.
11. Suhler EB, Lauer AK, Rosenbaum JT. Prevalence of serologic evidence of cat-scratch disease in patients with neuroretinitis. Ophthalmology 2000;107;871-876.
12. Drancourt M, Berger P, Terrada C, et al. High Prevalence of Fastidious Bacteria in 1,520 Cases of Uveitis of Unkown Etiology. Medicine 2008;87:167-176.
13. Ormerod LD, Dailey JP. Ocular manifestations of cat-scratch disease. Curr Opin Ophthalmol 1999;10:209–216.
14. Wade NK, Levi L, Jones MR, et al. Am J Ophthalmol 2000 Sep;130:327-34.
15. Wade NK, Po S, Wong IG, et al. Bilateral Bartonella-associated neuroretinitis. Retina 1999;19(4):355-6.
16. Mason JO. Retinal and Optic Nerve Neovascularization Associated with Cat Scratch Disease. Retina 2004;24:176-8.
17. Ziemssen F, Bartz-Schmidt K , Geliskenet F. Secondary Unilateral Glaucoma and Neuroretinitis: Atypical Manifestations of Cat-Scratch Disease. Jpn J Ophthalmol 2006;50(2):177-9.
18. Rehman SU, Metcalfe TW. Anterior uveitis associated with cat scratch disease. Br J Ophthalmol 1998;82:587–588
19. Reed JB, Scales DK, Wong MT, et al. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology 1998;105:459–466.
20. Ormerod LD, Skolnick KA, Menosky MM, et al. Retinal and choroidal manifestations of cat-scratch disease. Ophthalmology 1998;105:1024–1031.
21. Solley WA, Martin DF, Newman NJ et al. Cat Scratch Disease: Posterior Segment Manifestations. Ophthalmology 1999;106:1546-1553.
22. Jones MR, Cunningham ET Jr. Bartonella henselae-associated acute multifocal retinitis in a patient with acquired immunodeficiency syndrome. Retina 1997;17(5):457-9.
23. Chang MS, Lee SS, Cunningham ET Jr. Focal retinal phlebitis as a presenting sign of systemic Bartonella henselae infection. Retina 2001;21(3):280-1.
24. Cunningham ET Jr., McDonald HR, Schatz H, et al. Inflammatory mass of the optic nerve head associated with systemic Bartonella henselae infection. Arch Ophthalmol 1997;115:1596-7.
25. Rothova A, Kerkhoff F, Hooft HJ, et al. Bartonella serology for patients with intraocular inflammatory disease. Retina 1998;18:348-55.
26. Martinez-Osorio H, Calonge M, Torres J, et al. Cat-scratch disease (ocular bartonellosis) presenting as bilateral recurrent iridocyclitis. Clin Infect Dis 2005;40:E43–E45.
27. Jackson LA, Spach DH. Emergence of Bartonella quintana Infection among Homeless Persons. Emerging Infectious Diseases 1996;2:141-4.
28. Pape M, Kollaras P, Mandravelli K, et al. Occurrence of Bartonella henselae and Bartonella quintana among Human Immunodeficiency Virus-Infected Patients. Ann. NY Acad Sci 12005;063:299–30.
29. Warren K, Goldstein E, Hung VS, et al. Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch Ophthalmol 1998;116:937–940.
30. Curi AL, Machado DO, Heringer G, et al. Ocular manifestation of cat-scratch disease in HIV-positive patients. Am J Ophthalmol 2006;141:400–401.
31. Dehio C. Recent progress in understanding Bartonella-induced vascular proliferation. Current Opinion in Microbiology 2003;6:61-5.
32. Curi ALL, Campos WR, Barbosa L, et al. Unusual presentation of cat scratch disease in HIV+ patient. Br J Ophthalmol 2003;87:371.
33. Kerkhoff FT, Rothova A. Bartonella henselae associated uveitis and HLA-B27. Br J Ophthalmol 2000;84:1125–1129.
34. Drancourt M, Bodaghi B, Lepidi H, et al. Intraocular detection of Bartonella henselae in a patient with HLA-B27 uveitis. J Clin Microbiol 2004;42:1822–1825.
35. Vermeulen MJ, Herremans M, Verbakel H, et al. Serological testing for Bartonella henselae infections in The Netherlands: clinical evaluation of immunofluorescence assay and ELISA. Clinical Microbiology and Infection 2007;13:627-34.
36. Cunningham ET Jr., Koehler JE. Ocular Bartonellosis. Am J Ophthalmol 2000;130:340-349.
37. Roe RH, Jumper JM, Fu AD, Johnson RN, McDonald HR, and Cunningham ET Jr. Ocular Bartonella Infections. Int Ophthalmol Clin 2008; 48(3):93-105.