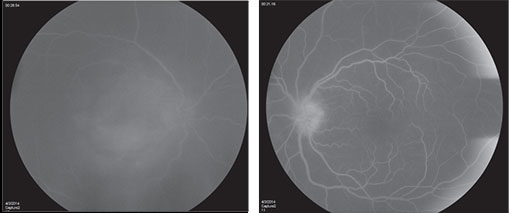
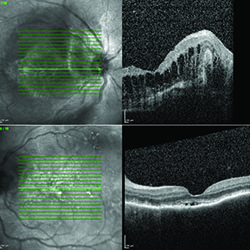
In the setting of extensive panuveitis in both eyes, the differential diagnosis for this individual was broad, including infection (tuberculosis, syphilis, Lyme disease, progressive outer retinal necrosis (PORN), and toxoplasmosis); inflammatory disease (serpiginous choroidopathy, multifocal choroiditis with panuveitis (MCP) and sarcoidosis); and neoplastic disorders such as primary intraocular lymphoma. Vascular causes, such as polypoidal choroidal vasculopathy and exudative age-related macular degeneration were considered less likely. Fluorescein angiography (See Figure 2) and optical coherence tomography (See Figure 3) were performed to assist in differentiating between clinical entities.
Despite having lived in the United States for 34 years, with her most recent trip to India 14 years ago, tuberculosis was high on our differential. She was started on prednisolone acetate 1% eye drops every hour in both eyes, and a uveitis workup was performed including complete blood count; comprehensive metabolic panel; QuantiFERON-TB Gold (QFT) assay (Qiagen, Germantown, Md.); and chest X-ray. The workup was negative, including chest X-ray, except for a positive QFT, consistent with tuberculosis.
Our patient was kept on frequent topical corticosteroid drops and it was recommended that she start a four-drug, anti-tuberculosis therapy (ATT) regimen of isoniazid, pyrazinamide, ethambutol and rifampin. She was referred to an infectious disease specialist for close management. The patient initially declined the ATT, wanting a second opinion for the diagnosis of TB chorioretinitis. She ultimately began taking the ATT once the vision in her left, better-seeing eye, declined to 20/200. One month after initiation of therapy her inflammation had begun to improve and visual acuity improved to 20/60 OS.
| ||||||||||||||
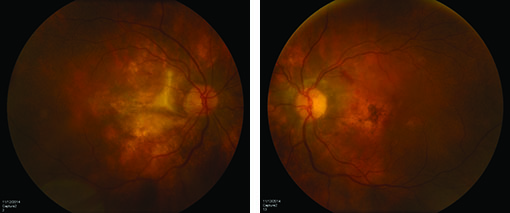
Oral corticosteroids were never started in this patient due to a new diagnosis of endometrial cancer, which was made in the first month after initiating ATT, and for which she underwent a hysterectomy. Her course was further complicated by development of a choroidal neovascular membrane OS for which she received an intraocular injection of bevacizumab with good response (See Figure 4).
Discussion
Infection by Mycobacterium tuberculosis can have manifestations in both the anterior and posterior segments of the eye. The most common ophthalmic manifestations of tuberculosis are uveitis (anterior, posterior or pan-uveitis), while posterior segment presentations include retinal vasculitis (primarily venous), optic neuritis, serpiginous-like choroiditis, and resulting choroidal and subretinal neovascularization.1 The anterior uveitis is typically “sticky,” with extensive posterior and peripheral anterior synechiae.2 Conjunctival granulomas are more common than lacrimal gland or orbital granulomas. Nodular scleritis and interstitial keratitis may occur. Choroidal granulomas or “tubercles” may be present as focal, elevated, dome-shaped lesions if direct invasion with bacteria occurs, and are especially prevalent in immunocompromised patients. Most diagnoses of TB-associated uveitis are presumptive, as the organism is difficult to directly isolate. Clinical criteria are used to make the diagnosis of TB-associated uveitis. The clinical criteria include a positive tuberculosis test (PPD or interferon gamma release assay such as QuantiFERON-TB Gold), clinical picture compatible with tuberculosis, response to treatment with ATT, or thorough complete diagnostic evaluation including microbiological isolation, X-ray, CT scan and/or MRI.3,4 It is essential to realize, however, that a majority of cases of ocular tuberculosis occur in the absence of any radiographic or symptomatic evidence of TB elsewhere.5
It has been estimated that nearly 2 billion people in the world are infected with Mycobacterium tuberculosis, 10 percent of whom will develop active TB at some point in their lifetime.6 Most cases of active tuberculosis, around 80 percent in the United States, are the result of reactivated latent infections.7 The incidence of latent tuberculosis in foreign-born persons living in the United States may be as high as 18.7 percent.8 In one study looking at all cases of presumed tuberculosis-induced scleritis and uveitis with positive QuantiFERON-TB Gold studies, 85 percent of patients had spent at least six months living in tuberculosis-endemic regions.9
Due to the high prevalence of disease in at-risk populations (e.g., immigrants from endemic areas, HIV-positive patients and people with prolonged exposure to prisons and homeless shelters), it is important to have a high index of suspicion in early cases of intraocular inflammation. A delay in the diagnosis of tuberculous eye disease is one of factors most associated with poor visual outcomes.5
The interferon gamma release assay (IGRA) demonstrates comparable sensitivity to the classic tuberculin skin test (TST) but is more specific in individuals who have previously received the BCG vaccine. Furthermore, IGRA tests do not require the patient to return for interpretation, making it more convenient. However, the IGRA tests are more expensive, and are not recommended for serial screening in patients with possible workplace exposure (e.g., health-care workers) because of a high rate of false-positive tests.
Two versions of this test are currently available: the QuantiFERON-TB Gold and T-SPOT TB tests.10 However, neither the IGRA nor TB skin testing will distinguish between latent TB infection and active TB. This test measures the response of a patient’s immune system to tuberculosis antigens. It is dependent on the patient’s immunologic status and may be falsely negative in patients with HIV infection, malignancy or iatrogenic immmunosuppression. There is no association between IGRA level and clinical disease severity, so the test is not useful for clinical monitoring or treatment response.9
Uveitis due to tuberculosis responds favorably to anti-tuberculosis therapy with complete remission rates reported as high as 91 percent.9 The initiation of therapy often includes a four-drug regimen of isoniazid, rifampicin, pyrazinamide and ethambutol, followed by a choice of medication combinations over the following four to seven months, which should be directed by an internist or infectious disease specialist.2 Following the initiation of ATT, a paradoxical worsening of uveitis and fundus appearance may occur. This clinical deterioration may represent a Jarisch-Herxheimer reaction, which has been speculated to derive from endotoxin release, delayed hypersensitivity or decreased suppressor mechanisms.11 The same phenomenon can be observed in systemic manifestations of tuberculosis. The possibility of a JHR emphasizes the utility of topical and/or systemic steroids on initiation of ATT.2 The use of corticosteroids in the treatment of ocular TB is unclear, with some authors reporting better outcomes while others report a higher risk of relapse.2,5
The diagnosis and treatment of TB-associated intraocular inflammation continues to be an evolving field as we understand more about the pathogenesis of the disease and concurrently improve accuracy of diagnosis. Practitioners should continue to be vigilant in considering tuberculosis in the differential diagnosis of uveitis and retinal vasculitis to ensure prompt initiation of therapy and to improve visual outcomes. REVIEW
1. Gupta V, Shoughy SS, Mahajan S, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm 2015;23(1):14-24
2. Gupta A, Bansal R, Gupta V, et al. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol 2010;149:562-570
3. Gupta V et al. Intraocular tuberculosis – An Update. Surv Ophthalmol 2007;52(6):561-80.
4. Ang M, Hedayatfar A, Zhang R, Chee SP. Clinical signs of uveitis associated with latent tuberculosis. Clin Experiment Ophthalmol 2012; 40: 689-696.
5. Patel SS, Saraiya NV, Tessler HH, Goldstein DA. Mycobacterial Ocular Inflammation: Delay in diagnosis and other factors impacting morbidity. JAMA Ophthalmol 2013;131:752-758.
6. Dye C et al. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282(7):677-86.
7. Horsburgh CR Jr, Rubin EJ. Clinical practice. Latent tuberculosis infection in the United States. N Engl J Med 2011;364(15):1441-1448.
8. Bennett DE, Courval JM, Onorato I, et al. Prevalence of tuberculosis infection in the United States population: The National Health and Nutrition Examination Survey, 1999-2000. Am J Respir Crit Care Med 2008;177:348-55.
9. La Distia N, van Velthoven M, ten Dam-van Loon N, Misotten T, et al. Clinical Manifestations of Patients With Intraocular Inflammation and Positive QuantiFERON–TB Gold In-Tube Test in a Country Nonendemic for Tuberculosis. Am J Ophthalmol 2014;157:754-761.
10. Dorman SE, Belknap R, Graviss EA, et al. Tuberculosis Epidemiologic Studies Consortium. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 2014;189(1):77-87.
11. Cheung CMG and Chee SP. Jarisch–Herxheimer reaction: Paradoxical worsening of tuberculosis chorioretinitis following initiation of antituberculous therapy. Eye 2009; 23:1472-1473.