|
Background
RP is an outer retinal degeneration that affects the retinal pigment epithelium and the photoreceptors. Eyes with RP respond to electrical stimulation because in many patients, the inner retina and ganglion cell layer still have some function. The retinal chip implants stimulate these cells.
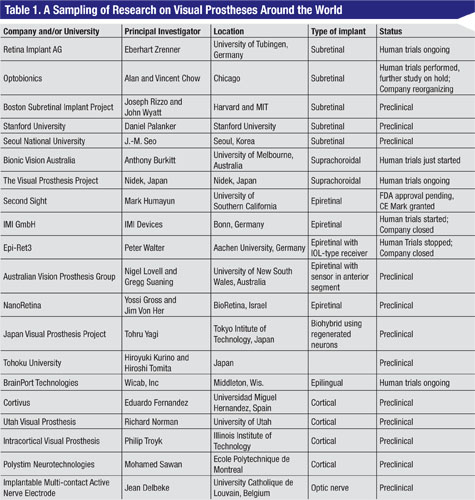
More than a dozen groups of investigators and companies around the world are working on retinal implants (Table 1). In order to restore visual function, chip implants have to detect light, convert the light energy to electrical energy, and then stimulate the retina. Different groups approach this in different ways.1,2 This article focuses on two implants that are furthest along the path to clinical availability: the Argus II Implant by Second Sight and the Active Subretinal Implant by Retinal Implant AG. The Argus Implant directly stimulates the ganglion cells. The Active Subretinal Implant recreates some of the signals that normally would have been made by the photoreceptors.
The Argus II Implant
Developed by Mark Humayun, MD, and colleagues at the Doheny Eye Institute, University of Southern California, the Argus II Implant has received a CE Mark in Europe and, this month, was granted approval by the U.S. Food and Drug Administration (See Review News, p. 3). The Argus II epiretinal implant is a 60-channel electrode array that directly stimulates the ganglion cells.3
The Argus II Implant consists of four parts. The power comes from a battery pack worn on the hip. An external video camera wirelessly delivers images to the electrical housing that is affixed to the episclera (similar in some sense to the plate of a glaucoma filtering tube). The image and data processing are done here. A cable from the electrical housing enters the eye through an incision in the pars plana and the electrical impulses then are sent through the cable to the chip. The chip itself is attached to the retina with a tack (See Figure 1A).
All 30 patients who received the implant during the trial were able to perceive light during stimulation. More than half of the patients were able to see the motion of a white bar moving across a black background. Many of the implanted patients were able to identify some 3 to 4.5 cm letters on a high-contrast background. The best vision to date was 20/1,262. These initial results are encouraging and will allow further refinement of the device.
|
One of the advantages of this implant is that it attaches directly to the ganglion cells, which are ultimately the cells that need to be stimulated to generate a visual signal that is then sent to the brain. As the electrode array becomes larger and/or is able to contain more electrodes, the cable might be able to be exchanged while keeping the electronic housing intact. From the patient’s perspective, the main disadvantage is the use of an external camera. To scan an area, the patient has to move the camera (basically his head) around. The current implant has a limited number of electrodes so the images only have rudimentary shapes, but this will be improved in future iterations.
The Active Subretinal Implant
Developed by Eberhard Zrenner, MD, and colleagues at the University of Tubingen in Germany, the Active Subretinal Implant is currently in clinical trials in several European centers and in Asia. The device is under review by the FDA as an investigational device and once FDA approval is obtained, Wills Eye Institute will be the lead clinical trial site in the United States. This implant contains a 1,500-electrode array that directly stimulates the inner retina. In contrast to the Argus II implant, which bypasses the inner retina, the Active Subretinal Implant aims to replace the dysfunctional photoreceptors.
The Active Subretinal Implant contains photodiodes on the subretinal chip, so there is no camera. The light stimulation occurs similar to the way we see—the light coming from an object goes through the pupil and activates the implant, which then converts the light directly into electrical stimulation. In contrast to the epiretinal implant, the subretinal implant does the image processing within the chip itself. However, using this technology requires more energy than light can provide. This is provided via a handheld battery pack that also has controls for brightness and contrast. The necessary energy is transmitted transdermally via a receiver induction coil and a magnet that is implanted under the skin behind the ear. A subdermal cable tacked to the lateral canthus connects the receiver to the subretinal implant for energy.
| ||||||
|
Although both the Argus II Implant and the Active Subretinal Implant surgeries are somewhat complicated, both use techniques familiar to vitreoretinal surgeons.
The ability to integrate retinal implant technology into the human neural system to restore limited vision has not only been a major scientific advance, but also a positive life-changing experience for many of the selected patients in these limited clinical trials. The results of these trials, current and future engineering and technical advances, and the evolving adaptive ability of patients learning to resume normal activities of daily living offer hope for our blind patients. REVIEW
Dr. Garg is an associate professor of ophthalmology at the Retina Service of Wills Eye Institute, and is a partner with MidAtlantic Retina. He can be reached at
sgarg@midatlanticretina.com.
1. Weiland JD, Cho AK, Humayun MS. Retinal Prosthesis: current clinical results and future needs. Ophthalmology 2011;118:2227-37.
2. Rizzo JF, Shire DB, Kelly SK, et al. Overview of the Boston Retinal Prosthesis: Challenges and opportunities to restore useful vision to the blind. Conf Proc IEEE Eng Med Biol Soc 2011;2011:7492-5.
3. Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 2012;119:779-88.
4. Wilke R, Gabel VP, Sachs H, Bartz Schmidt KU, et al. Spatial resolution and perception of patterns mediated by a subretinal 16-electrode array in patients blinded by hereditary retinal dystrophies. Invest Ophthalmol Vis Sci 201129;52(8):5995-6003.
5. Zrenner E, Bartz-Schmidt JU, Benav H, et al. Subretinal electronic chips allow blind patients to read letters and combine them into words. Proc Biol Sci 2011 May 22;278(1711):1489-97.