The Need for Prostheses
There are a variety of diseases that target the visual pathway, with retinitis pigmentosa and age-related macular degeneration being two of the leading causes of photodegenerative blindness in the United States.1
Retinitis pigmentosa is a complex genetic disease typically characterized by retinal hyperpigmentation and photoreceptor death. Retinitis pigmentosa initially affects rod cells and low-contrast peripheral vision, while sparing high-acuity central vision. In more severe forms of the disease, however, visual acuity can fall to 20/200 (25 percent of cases) or zero light sensitivity (0.5 percent of cases) due to damage to macular cones.2,3
 |
While AMD is also characterized by photoreceptor death, it typically results in the progressive loss of central visual acuity due to cone cell damage. Age-related macular degeneration is the leading cause of progressive blindness in the elderly.
Though anti-vascular endothelial growth factor treatments, as well as photodynamic therapy, have helped minimize disease progression in AMD,4,5 there are few available options for restoration of functional eyesight in patients who have significant photoreceptor loss. Several approaches, however, including retinal/visual prosthesis implantation, optogenetic stimulation and stem cell therapy, are currently being developed to restore some level of functional vision in patients suffering from these conditions.
Early Visual Prostheses
The earliest forays into visual stimulation began in 1918 with the work of Lowenstein and Borchardt, who elicited flickering sensations of light through occipital stimulation in a projectile-wound patient.6 Similar results were obtained by Krause in 1928 and Foerster in 1929, who conducted further stimulation studies in projectile-wound patients. Their experiments produced phosphenes of light in predictable, retinotopic patterns in blind or hemianopic patients and suggested that future prototypes could potentially allow patients to avoid obstacles when walking, and to read at speeds comparable to sighted individuals.6
These findings led to the development of early prostheses that were tested in humans by Brindley and Lewin in 1968, who examined retinotopy, phosphene threshold, pulse duration and frequency requirements in a bilateral glaucoma patient using an 80-electrode platinum array.6,7 Later prostheses developed by Dobelle (1974) and Normann (1989) expanded on this concept and were able to test high-density electrode arrays in sighted and blind patients.8-10
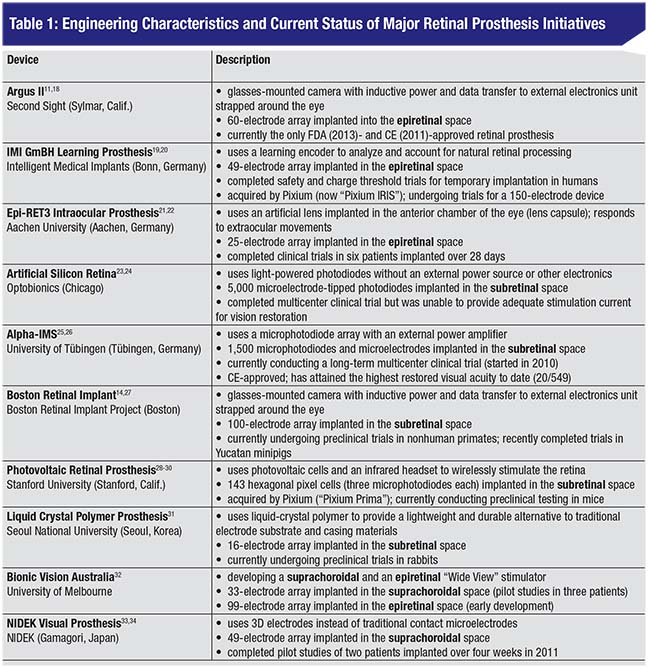
Applications Using the Retina
While the occipital lobe has the advantage of being a large, accessible target for stimulation, a great deal of processing occurs between the retina and the visual cortex. Hence, recent focus has shifted towards points in the visual pathway such as the retina, optic nerve and lateral geniculate nucleus of the thalamus. The development of retinal prosthetics has seen remarkable growth over the past 20 years due to surgical accessibility, technological advances driven by the semiconductor industry and the lack of complex processing in the retina compared to later points in the visual pathway.
A retinal prosthesis is often composed of three key components (See Figure 1): an image capturing unit; a video-processing unit; and a microelectrode array. The image-capturing unit, often a glasses-mounted video camera or microphotodiode array, takes light information and transforms it into electrical information much as photoreceptors do. Information is transmitted from the camera to a video-processing unit, often worn on the patient’s belt, which compresses and simplifies complex image information into points of electrical stimulation. The VPU transmits this information via induction, light or wire to electronics implanted in and around the eye, which receive the encoded stimulation data and directly activate the retina.
Retinal approaches are often organized based on stimulation target. (See Figure 2) Microelectrode arrays are typically implanted
 |
| Figure 1. Typical components of a retinal prosthesis: an image-capturing unit; a video-processing unit; an external transmitter; an implanted electronics case; and a microelectrode array. Other alternative designs include the use of infrared power and data transfer, microphotodiode arrays for light capture and implantation of the electronics case into the lens capsule. |
Epiretinal stimulation, for example, allows for heat dissipation through the vitreous and implantation of larger arrays through a pars plana vitrectomy; however, it also requires stabilization with a retinal tack which may result in array dislodgement or gliosis.11-13 Since it directly stimulates the retinal ganglion cells, this approach may also require more advanced image processing.13
In contrast, subretinal stimulation directly targets the bipolar neurons and doesn’t use tack fixation, but instead requires the use of trans-scleral cables and specialized surgery involving injection of a subretinal saline bleb.12-14 Though suprachoroidal approaches require a less-invasive surgical procedure, they result in a greater distance between the electrode array and retinal tissue, which may limit the potential for high-acuity restoration.13
Our group recently reviewed and conducted an engineering analysis of 10 retinal prosthesis initiatives.15 (See Table 1) Of these, four have recently completed human trials (Argus II, IMI GmBH, Epi-Ret3 and ASR), three are undergoing single- or multicenter human trials (Alpha-IMS, BVA and Nidek) and three are undergoing preclinical animal studies (BRIP, Photovoltaic and LCP). Prostheses typically use microphotodiodes or external glasses-mounted cameras for image capture, light-based or inductive power and data transfer, and a microelectrode array composed of a polymer substrate and platinum or iridium oxide electrodes.1,11,16,17 Only the Argus II has been approved by the Food and Drug Administration, and both the Argus II and the Alpha-IMS have been approved for commercial use in Europe. The Alpha-IMS has attained the highest visual acuity to date using a Landolt-C test (20/546), and is the only device currently undergoing a multicenter clinical trial.17
Retinal prostheses have demonstrated significant progress in improving visual acuity and activities of daily living in blind patients. Several initiatives reported that patients were able to navigate their surroundings, identify objects and read large letters using implants or simulations.25,35,36 However, retinal stimulation requires surviving bipolar and ganglion cell targets within the retina. Though photodegenerative diseases like RP and AMD leave the inner layers of the retina relatively intact, other common conditions such as glaucoma, ocular trauma and optic neuritis damage the retina or optic nerve, precluding the use of retinal prosthetics.12 To this end, several initiatives (shown in Table 2) are also conducting research on alternative targets for electrical stimulation (visual cortex, thalamus and optic nerve) and non-electrical stimulation (optogenetic and neurotransmitter titration).
Future Directions
The goals of retinal and visual prosthesis initiatives have evolved significantly over time. What began as an early 20th century exploration of the occipital cortex in seizure and projectile-wound patients led to the early development of visual prostheses like the Dobelle eye and the Artificial Silicon Retina, which provided the foundation for future work in restoring vision through visual prosthetics. As researchers develop advanced technology and a greater understanding of the visual system, prosthetic research will continue to advance, resulting in increased implant size and electrode density and improved device biocompatibility and surgical implantation protocols.
 |
To date, more than 100 patients have been acutely or chronically implanted with retinal prostheses. In simulations and long-term studies, patients have experienced improvements in both visual acuity and quality of life. While visual resolution is low, patients are able to do such things as locate bright objects on a dark table, discern grating patterns and identify simple objects including fruit, utensils and geometric shapes.25 Implanted patients were also able to navigate a room and scored higher on the Functional Low-Vision Observer Rated Assessment, a quality-of-life assessment developed by the FDA for retinal prostheses.37
Despite these advances, visual prosthetics face several engineering obstacles. Ways to measure the success of a prosthesis include visual restoration and biocompatibility. Visual restoration refers to the ability of prostheses to improve a patient’s visual acuity and visual field, while biocompatibility is reflected in the number of severe adverse events experienced by patients.
Visual acuity can be influenced by several factors, including electrode number and density, electrode overlap, understanding of intrinsic retinal processing and psychophysical performance. While the Alpha-IMS implant has demonstrated the highest visual acuity to-date (20/546), most prostheses are only able to obtain a visual acuity of 20/1000 or worse. It’s believed that upwards of 600 to 1,000 pixels are required for restoration of useful vision; however, the majority of prostheses use 100 or fewer stimulating electrodes.11 It’s also difficult to reduce electrode size without raising charge densities to unsafe levels.38 More research is also needed on visual processing in the retina and psychophysical performance associated with retinal stimulation.
In contrast, visual field restoration is often correlated with array size.
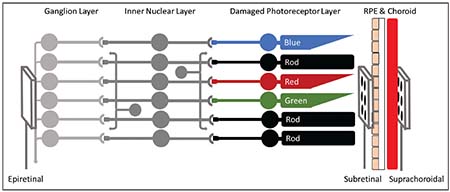 |
| Figure 2. A brief overview of retinal stimulation targets: Photoreceptors in the retina transform light information into electrical and chemical signals. These signals are passed through the inner nuclear layer (bipolar cells, horizontal cells and amacrine cells) to reach the ganglion cell layer, which fires action potentials down the optic nerve. Stimulating arrays are typically implanted in the epiretinal space (between the vitreous and RGCs), in the subretinal space (between the damaged photoreceptors and retinal pigment epithelium) or in the suprachoroidal space (outside the choroid and beneath the sclera). |
Biocompatibility is crucial for safety. While most prostheses have demonstrated a relatively low frequency of SAEs in humans, few studies have been conducted on the effects of chronic implantation.1,18,39 However, some engineering characteristics that may play roles in biocompatibility include implant material, charge density, invasiveness and disease-induced change. While studies have been conducted on optimal materials for stimulation and implantation,40 further long-term implantation studies in the retina are needed. Disease-induced changes in retinal physiology, separation of electrode-tissue interfaces and material considerations may also cause high charge densities, damage and/or gliosis.
Visual prosthesis research has made significant progress in improving the daily lives of patients suffering from diseases like RP and AMD. While several engineering limitations and challenges facing the field of retinal and visual prosthetics remain, recent advances in technology and physiology may soon lead to the use of prosthetics as a viable treatment for photodegenerative diseases. REVIEW
Derrick Cheng is a medical student at the Warren Alpert Medical School of Brown University. Dr. Borton is assistant professor of engineering at Brown University and the Brown Institute for Brain Science. Dr. Greenberg is professor of surgery (ophthalmology) at the Warren Alpert Medical School, and is chief of ophthalmology at the Providence VA Medical Center. Dr. Greenberg can be reached at paul_greenberg@brown.edu.
The authors declare no financial interest in any product discussed.
The views expressed in this article are those of the authors and don’t necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. Government.
1. Matthaei M, Zeitz O, Keserü M, et al. Progress in the development of vision prostheses. Ophthalmologica 2011;225:4:187-92.
2. Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A 2006;103:30:11300-5.
3. Grover S, Fishman GA, Anderson RJ, et al. Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology 1999;106:9:1780-1785.
4. Musarella MA, Macdonald IM. Current concepts in the treatment of retinitis pigmentosa. J Ophthalmol 2011:753547.
5. Imrie FR, Bailey C. New treatments for age-related macular degeneration. Age Ageing 2007;36:1:8-10.
6. Lewis PM, Rosenfeld JV. Electrical stimulation of the brain and the development of cortical visual prostheses: An historical perspective. Brain Res 2016;1630:208-24.
7. Brindley GS, Lewin WS. The sensations produced by electrical stimulation of the visual cortex. J Physiol 1968;196:479-493.
8. Dobelle WH, Mladehovsky MG. Phosphenes produced by electrical stimulation of human occipital cortex and their application to the development of a prosthesis for the blind. J Physiol 1974;243:553-576.
9. Dobelle WH. Willem J. Kolff and artificial vision for the blind. Artif Organs 1998;22:11:966-968.
10. Normann RA, Maynard EM, Rousche PJ, Warren DJ. A neural interface for a cortical vision prosthesis. Vis Res 1999;39:15:2577-2587.
11. Weiland JD, Cho AK, Humayun MS. Retinal prostheses: current clinical results and future needs. Ophthalmology 2011;118:11:2227-37.
12. Margalit E, Maia M, Weiland JD, et al. Retinal Prosthesis for the Blind. Surv Ophthalmol 2002;47:4:335-356.
13. Weiland JD, Humayun MS. Retinal Prosthesis. In: He B, ed. Neural Engineering. New York: Kluwer Academic/Plenum, 2013:635-655.
14. Rizzo JF. Update on retinal prosthetic research: The Boston Retinal Implant Project. J Neuroophthalmol 2011;31:2:160-8.
15. Cheng DL, Greenberg PB, Borton DA. Advances in retinal prosthetic research: A systematic review of engineering and clinical characteristics of current prosthetic initiatives. Current Eye Research 2017:1-14.
16. Luo YH, da Cruz L. A review and update on the current status of retinal prostheses (bionic eye). Br Med Bull 2014;109:31-44.
17. Chuang AT, Margo CE, Greenberg PB. Retinal implants: A systematic review. Br J Ophthalmol 2014;98:7:852-6.
18. Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 2012;119:4:779-88.
19. Eckmiller R, Neumann D, Baruth O. Tunable retina encoders for retina implants: Why and how. J Neural Eng 2005;2:1:S91-S104.
20. Keseru M, Feucht M, Bornfeld N, et al. Acute electrical stimulation of the human retina with an epiretinal electrode array. Acta Ophthalmol 2012;90:1:e1-8.
21. Klauke S, Goertz M, Rein S, et al. Stimulation with a wireless intraocular epiretinal implant elicits visual percepts in blind humans. Invest Ophthalmol Vis Sci 2011;52:1:449-55.
22. Gerding H, Benner FP, Taneri S. Experimental implantation of epiretinal retina implants (EPI-RET) with an IOL-type receiver unit. J Neural Eng 2007;4:1:S38-49.
23. Pardue MT, Phillips MJ, Yin H, et al. Possible sources of neuroprotection following subretinal silicon chip implantation in RCS rats. J Neural Eng 2005;2:1:S39-47.
24. Chow AY, Chow VY, Packo KH, et al. The Artificial Silicon Retina Chip for the Treatment of Vision Loss. Arch Ophthalmol 2004;122:4:460-469.
25. Zrenner E, Bartz-Schmidt K, Benav H, et al. Subretinal electronic chips allow blind patients to read letters and combine them into words. Proc Biol Sci 2011;278:1711:1489-97.
26. Kitiratschky VB, Stingl K, Wilhelm B, et al. Safety evaluation of “retina implant alpha IMS”—A prospective clinical trial. Graefes Arch Clin Exp Ophthalmol 2015;253:3:381-7.
27. Kelly SK, Shire DB, Chen J, et al. A hermetic wireless subretinal neurostimulator for vision prostheses. IEEE Trans Biomed Eng 2011;58:11:3197-205.
28. Lorach H, Goetz G, Smith R, et al. Photovoltaic restoration of sight with high visual acuity. Nat Med 2015;21:5:476-82.
29. Wang L, Mathieson K, Kamins TI, et al. Photovoltaic retinal prosthesis: Implant fabrication and performance. J Neural Eng 2012;9:4:046014.
30. Palanker D, Vankov A, Huie P, Baccus S, et al. Design of a high-resolution optoelectronic retinal prosthesis. J Neural Eng 2005;2:1:S105-20.
31. Lee SW, Seo JM, Ha S, et al. Development of microelectrode arrays for artificial retinal implants using liquid crystal polymers. Invest Ophthalmol Vis Sci, 2009;50:12:5859-66.
32. Ayton LN, Blamey PJ, Guymer RH, et al. First in-human trial of a novel suprachoroidal retinal prosthesis. PLoS One 2014;9:12:1.
33. Ohta J, Tokuda T, Kagawa K, et al. Laboratory investigation of microelectronics-based stimulators for large-scale suprachoroidal transretinal stimulation. J Neural Eng 2007;4:1:S85-91.
34. Fujikado T, et al. Testing of semichronically implanted retinal prosthesis by suprachoroidal-transretinal stimulation in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci, 2011;52:7:4726-33.
35. Vurro M, Crowell AM, Pezaris JS. Simulation of thalamic prosthetic vision: Reading accuracy, speed, and acuity in sighted humans. Front Hum Neurosci 2014;8:816.
36. Dagnelie G, Keane P, Narla V, et al. Real and virtual mobility performance in simulated prosthetic vision. J Neural Eng 2007;4:1:S92-101.
37. Ho AC, Humayun MS, Dorn JD, et al. Long-term results from an epiretinal prosthesis to restore sight to the blind. Ophthalmology 2015;122:8:1547-54.
38. Cogan SF. Neural stimulation and recording electrodes. Annu Rev Biomed Eng 2008;10:275-309.
39. Stingl K, Bartz-Schmidt KU, Besch D, et al. Subretinal Visual Implant Alpha IMS—Clinical trial interim report. Vision Res 2015;111(Pt B):149-60.
40. Scholz C. Perspectives on: Materials aspects for retinal prostheses. Journal of Bioactive and Compatible Polymers 2007;22:5:539-568.



