Hereditary retinal dystrophies can do their worst just when patients are trying to live their fullest lives, leaving them with no useful vision at the most advanced stages. The Food and Drug Administration’s approval of Luxturna (Spark Therapeutics; Philadelphia), a gene therapy injected subretinally, offers hope to patients with mutations to the RPE65 gene. Retinal prosthetic devices are a concept that both pre-dates gene therapy and continues apace with it. Here’s an update on two such devices currently being implanted to address blindness from advanced retinitis pigmentosa.
The Argus II
The Argus II Retinal Prosthesis (Second Sight; Sylmar, Calif.), CEmarked in 2011 and FDA-approved for humanitarian use in 2013, consists of a pair of glasses with a video camera mounted in the center and an external coil on the sidearm, and a portable, battery-powered video processing unit that wirelessly transmits electronic pulses to a tiny, 60-channel electrode chip implanted epiretinally, via a cable that enters the eye from the housing of the chip. “The Argus II is a device that restores sight to patients with certain types of retinal blindness, particularly patients who suffer from inherited retinal degeneration that leads to loss of photoreceptors,” says Mark Humayun, MD, PhD, co-inventor of the Argus series of retinal implants.
“In addition to the wearable components, the implanted component is a computer chip that receives the information; the chip’s delicate electrode array converts that information into controlled electrical pulses that stimulate the ganglion cells in the retina and send the information to the optic nerve,” Dr. Humayun explains. “So it’s basically a camera system, wirelessly connected to implanted electronics, that jumpstarts the otherwise blind eye. It bypasses the function of the lost or damaged photoreceptors. It provides that information to the retina, and then the retina relays it to the brain.”
According to Dr. Humayun, the success of the cochlear implant for deafness was a source of inspiration for the Argus retinal implant. “Wireless links like the one in Argus have been used extensively in cochlear implants and elsewhere in the body. We have decades of experience with that technology,” he says.
Dr. Humayun says that the implantation procedure for the Argus II is well within the skill set of a retinatrained surgeon. “Most of the steps of the surgical procedure could easily be done by a retinal surgeon,” he says. “There are certain parts, such as attaching the electrode array to the retina, that the surgeon has to learn.” The surgeon must create a vitreous detachment to insert the electrode array epiretinally, and affixing it in place is one tricky element of the procedure, he says. “You have to use a tiny tack to attach the chip to the back of the eye,” he notes. “That’s a step you normally don’t do, but it’s easily learned. More than 200 patients have been implanted in 30 countries worldwide, including Europe, the United States, Canada, the Asia Pacific region, the Middle East, Russia and even Iran.”
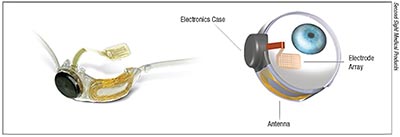 |
| The ocular-implant component of the Argus II Retinal Prosthesis System, outside and inside the eye. The sheet of microelectrodes receives information from the antenna, which gets the signal from a camera housed in the recipient’s glasses after an external, wearable video-processing unit processes the original image captured by the glasses-mounted camera. The electrodes then emit pulses that bypass damaged photoreceptors to stimulate remaining cells in the retina, which transmit the pulses to the optic nerve and then the brain. Patients must learn how to interpret the pulses as visual patterns of light, contrast and pixelated shapes. |
In a study of 30 blind retinitis pigmentosa patients implanted with the Argus II and followed for 36 months, all those who reported that their vision negatively affected their quality of life with respect to getting injured, meeting life’s demands and fulfilling life roles reported improvement across those dimensions.1
“Whether it occurs at the retinal level or at the brain level, adaptation is key [to these improvements],” says Dr. Humayun. “When we implant the device we see a learning effect. The patient is now using the eye’s remaining cells after perhaps decades of complete blindness, and suddenly getting used to this type of stimulation. It’s very different from what we normally see, because electrodes from the chip are stimulating groups of neurons; whereas light can stimulate single rods and cones.
“In the eye, initially the input is different, maybe just spots of light,” he continues. “Eventually, however, the patient learns to use the signals to recognize objects.” after Argus implant surgery, patients may be able to see light and motion, and may also become able to do things like sort laundry or follow lines in crosswalks and avoid obstacles while walking; some may even be able to read very large letters close up or sort laundry visually, according to the Second Sight website’s FAQ page for patients (secondsight.com). Dr. Humayun says that the Argus II’s software code is more sophisticated and better presented than in the earlier version of the device, and is therefore more “readable” by the patient, resulting in meaningful visual gains sooner for those who take the time to practice with the system after implantation. “We didn’t fully understand the code early on, but as we understand it more and we know how to present it better, the acclimation process is down to months, as opposed to years,” he notes.
Retina Implant Alpha AMS
The Retina Implant Alpha AMS (Retina Implant AG; Reutlingen, Germany), CE-marked in 2013, is an investigational device in the United States, and is currently intended to treat patients with blindness due to retinitis pigmentosa. The Alpha AMS doesn’t require an external camera. It’s a 1,600-pixel microphotodiode array implanted subretinally, relying on light to stimulate the optic nerve via remaining RPE cells.
“In retinitis pigmentosa, when the retinal pigment epithelium sustains damage, those cells are no longer able to take care of the rods and the cones, and the rods and the cones and the photoreceptors get damaged,” explains Sunir J. Garg, MD, principal investigator and chief surgeon for the current feasibility trial (ClinicalTrials.gov Identifier: NCT03629899) at Wills Eye Hospital in Philadelphia. “The chip implant goes underneath the retina, with the photodiodes directly touching the rod and cone cells. An electrical signal stimulates those rods and cones. We’re basically replacing or supplementing the RPE cells, helping to rev up the cells that are still functional but weakened. Then the electrical signals go through the normal pathway: through the retina and the optic nerve and to the brain.”
The implant includes a handheld component about the size of a cellphone that holds batteries and transmits energy via magnetic induction to a ceramic-housed coil implanted under the skin behind the ear. The patient can also adjust for brightness and contrast using the handheld device.“They can tweak it, but some of the tweaking is also done by us in our laboratory,” says Dr. Garg.
Since the Alpha AMS doesn’t require an external camera to gather information, patients glance around by moving their eyes, rather relying on head movement to accommodate a limited visual field. “One of the neat things about this particular implant is that there’s no camera,” Dr. Garg says. “The patient is looking through his or her own eye; light’s coming in through the pupil just like it always did. It’s being focused on the retina just like it always has been. Once light hits the implant, the implant does what the damaged cells can’t do, which is provide stimuli.”
 |
| Close-up view of the photodiode array of the RI Alpha AMS. The array replaces degenerated RPE. The chip is 3.2 x 4 mm and 70 μm thick. The photodiodes convert light that enters the eye into an electrical signal that the functional retinal cells amplify; and the signal then travels through the optic nerve to the brain. |
As with the Argus II, Dr. Garg says that retinal surgeons can master the skills needed to implant the Alpha AMS. “The implantation techniques are essentially techniques that vitreoretinal surgeons are comfortable with, including vitrectomy, creating a retinal detachment and doing scleral cutdowns.” There are a few caveats, though. “The surgical skills are combined in a way that’s very different than what we’re used to, however. In order to get comfortable with the procedure, I think people will have to spend time in a training laboratory setting, under the mentorship of people who’ve done it several times,” Dr. Garg continues. “It’s not as simple as watching a video once or twice and figuring that you can do it. It does require dedicated time under supervision in a practice lab to learn.”
The other added consideration when implanting an Alpha AMS is that the procedure takes what Dr. Garg calls “one big OR day” working together with colleagues from other surgical specialties. “There’s a battery pack that’s implanted behind the ear, similar to the way a cochlear implant battery pack goes behind the ear: That requires the expertise of an ENT surgeon or a neurosurgeon, just because they’re the ones with experience doing cochlear implants. There’s also wire that’s tunneled from behind the ear to the orbit; that wire is placed underneath the skin. An oculoplastics person generally does that. You need a team of experts working together. Some facilities may be more readily equipped than others to assemble that team,” he acknowledges.
Although Dr. Garg and colleagues haven’t yet implanted any patients with the device, he reports a lot of interest. He hopes to operate on his first patient by the end of 2018 or early 2019, although other patients have received the device outside of the United States. “From a clinical perspective, we’re looking for patients who have either bare light perception or no light perception,” he says of the screening process. “But what’s interesting about these patients is that even though they have no light perception when we measure in the clinic, they can still have some of their retinal cells functioning; and if you do electrical stimulation studies and ultra low-vision testing, you can still detect that the retina is functional. Although we’re looking at people who have profound vision loss, we want to make sure the retina is at least somewhat functional for them to be in the trial. We’re also currently working with patients who are middle-aged (50 years) or older.”
The Retina Implant Alpha AMS seems to help end-stage RP patients with their light perception and high-contrast object recognition. Five of six Alpha AMS recipients demonstrated improved visual functioning with the implant turned on versus off for up to 24 months. (One patient had to be explanted at three months due to iatrogenic damage to the device and incorrect implantation.)2
“We’re still learning how all the neurons and synapses interact with each other,” Dr. Garg notes. “I guess I’m surprised—but not too surprised—to see that if you reestablish pathways that have been dormant for awhile, the body is going to try to make the best of the hand that it’s been dealt. So this device is pretty good. Although I’ve seen some variable results, when patients have a good result, it can really be quite spectacular. These are people who’ve literally been in the dark for years. One patient who has received the implant is able to look at his child and see her face; he’s able to reach out and touch her nose. Another patient was able to notice that one of his kids was wearing a bracelet, and could reach out and touch it. The resolution’s clearly not as good as the Retina display in your iPhone, for example, but it’s definitely better than some of the pixelated images we’ve seen with some of the other technologies. So for some patients, I think it can really offer them meaningful visual perception and information that they haven’t had in a long time.”
Pros and Cons
Dr. Humayun and Dr. Garg both acknowledge that there are pros and cons to both retinal prostheses, with the placement of the electrode and photodiode arrays in the respective devices being a prime example. “The Argus II goes on top of the retina, so it’s stimulating the cells, but it’s doing so in a slightly different place than they would normally be stimulated. Therefore, the Alpha AMS implant may be more directly targeting the affected cells,” says Dr. Garg.
While the Alpha AMS more directly stimulates the photoreceptors by virtue of where it’s implanted, the cells most proximal to the implant may not be the best carriers of stimuli in the setting of disease, Dr. Humayun notes. “Theoretically, the subretinal device is closer to the next cell in line, which is potentially advantageous, but a lot of aberrant rewiring occurs in the retina. A subretinal device may end up stimulating the next cell in line, but it may be aberrantly rewired so you lose that benefit,” he says. “Whereas the epiretinal device stimulates the ganglion cells, which are less prone to rewiring.”
Some patients seeking to restore visual functioning through these technologies may find that they’re not suitable candidates for one type of implant or the other right out of the gate. Dr. Humayun contrasts the anatomical requirements for the Alpha AMS and the Argus II. “A photodiode array gets its information through light, so it requires a clear cornea and lens, which is not necessary for the Argus system to work,” he says.
“One of the other big things to keep in mind is that these devices are not suitable for everybody,” says Dr. Garg. “Patients have to really want to be involved in a trial, and have to want to be explorers who are willing to try something new to help drive the field forward and help us learn. That’s an important thing to keep in mind that we don’t traditionally think about with clinical trials. After the implant is done, patients have to spend a reasonable amount of time in rehabilitation, learning how to use the device and integrate it into their daily activities.”
Future Advances
Dr. Garg is optimistic that as retinal implants improve, surgeons will be able to help more patients to a greater degree. “My hope is that, just as computer technology keeps on getting better, faster, smaller and cheaper, we’ll be able to see those types of advances with these implants. As time goes on and we have more experience with the Alpha AMS trial and we overcome some of the technological limitations, I hope that the implants will keep getting better. There are certain diseases, such as geographic atrophy from macular degeneration or central vision loss from Stargardt’s disease, and others, that may benefit from these types of technologies in the future. We’re not there now, but I’m hopeful that things will improve faster and faster, to the point where we’ll be able to help those patients as well in the near future.”
To that end, a feasibility study of the Argus II is underway to evaluate its use in dry AMD patients (ClinicalTrials.gov Identifier: NCT02227498). Dr. Humayun also notes that Second Sight is launching a new device that may broaden the indications for the implantable devices by working around the retina and optic nerve altogether. “We just launched the Orion Visual Cortical Prosthesis System, a cortical implant device that goes directly into the vision center of the brain,” he reports. “So far, five subjects have been implanted. It still has a camera and wireless transmission. But instead of going into the retina, the electrodes go into the brain.”
The Orion won FDA clearance for a feasibility trial late last fall (ClinicalTrials.gov Identifier: NCT03344848). Because its electrode array goes on the surface of the visual cortex, it may be suitable for completely blind patients whose vision loss stems from trauma, DR, glaucoma or other conditions. REVIEW
Dr. Humayun holds equity in Second Sight and receives patent royalties for his work on the Argus Series I and II.
Dr. Garg is the principal investigator and chief surgeon of the current feasibility trial for the Retina Implant Alpha AMS, but receives no remuneration in this role.
1. Duncan IL, Richards TP, Arditi A, et al. Improvements in visionrelated quality of life in blind patients implanted with the Argus II Epiretinal Prosthesis. Clin Exp Optom2017;100:2:144-50.
2. Edwards TL, Cottriall CL, Xue K, et al. Assessment of the Electronic Retinal Implant Alpha AMS in restoring vision to blind patients with end-stage retinitis pigmentosa. Ophthalmology 2018;125:432-43.



