Meanwhile, ever-larger and better-constructed clinical trials, along with research, clinical observation and our growing knowledge of genetics, are expanding our understanding of the causes and mechanisms of glaucoma. However, as this evolution continues, the language we use when defining POAG and its subtypes is important. This is true because the assumptions built into our terms color our perceptions and expectations, as well as the research we choose to conduct and the treatment approaches we choose for our patients.
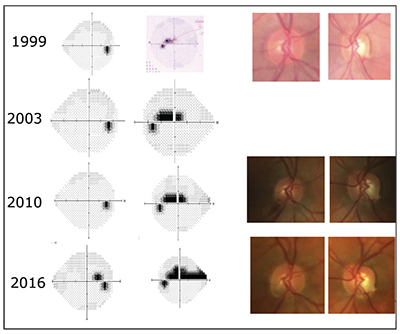 |
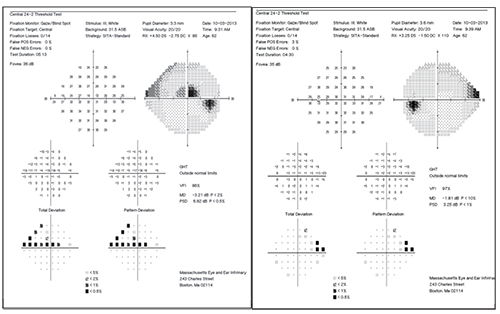
| This patient continued to progress despite pressures in the low teens, with damage focused near the center of vision. Her father had higher pressures (24 mmHg untreated) and had lost most of his vision to glaucoma. |
I believe our current terminology relating to POAG works against us by conflating and separating as-pects of glaucoma in ways that are counterproductive and not supported by the evidence. For example, I believe we need to avoid equating POAG with high-tension disease—in other words, disease that occurs only in people who have pressures greater than 21 mmHg. Clearly, POAG can occur across a broad spectrum of intraocular pressures. Using terms such as low-pressure or normal-tension glaucoma is also misleading; it suggests that these individuals and high-tension glaucoma patients have different diseases, when in fact they may have overlapping pathologic features.
Here, I’d
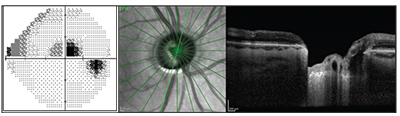 |
| Many patients fitting the description of paracentral open-angle glaucoma have a triangular prelaminar neuronal defect within the cup that can clearly be seen on OCT. |
Paracentral OAG
Consider a 39-year old patient who came to see me for the first time 17 years ago. She was referred by the people at LensCrafters, who—to their credit—noticed a disc hemorrhage on her left optic nerve. When I examined her, I saw the disc hemorrhage and observed that the left optic nerve had some vertical elongation of the cup. Yet her slit lamp examination was normal; her IOPs were 18 mmHg and her corneal thicknesses weren’t profoundly thin. To make matters even more interesting, I soon realized that her father had been my patient for the previous nine years; he had advanced primary open-angle glaucoma. However, his untreated IOPs were much higher—24 mmHg.
This raises a question: How can it be that my 39-year-old patient, who might be diagnosed as having “normal-tension glaucoma,” have a father who had “high-tension glaucoma?” The answer is most likely that the IOP we measure is not as important as the underlying genetic mechanisms of the disease. As you can see in the series of visual fields taken over the past 17 years, despite my attempts to keep her pressures in the low teens, she continues to progress. In particular, notice how her disease is relentlessly attacking the center of her vision.
I propose that one subtype of primary open-angle glaucoma is an entity
 |
• A distinct structural profile. Many of these patients have a triangular prelaminar neuronal defect within the cup that can be seen on OCT. It’s almost as if there’s a little man with a shovel digging up this discrete neuronal segment of the prelaminar neuronal tissue.
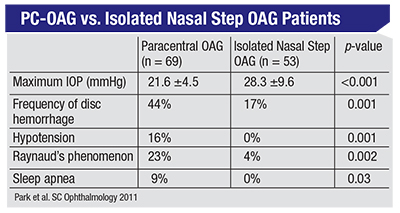
• Lower mean IOP. Compared to patients who have isolated nasal steps, patients with paracentral open-angle glaucoma tend to have a lower mean IOP. Consider the data from a 2011 study by Sung Chul Park, MD, and colleagues1. Note that the mean IOP in the paracentral open-angle glaucoma cases was 21.6 ±4.5 mmHg. This is important because it means that some of the patients with this disease had IOPs that were close to 16 mmHg, while others had IOPs close to 24 mmHg. This example helps to illustrate why the concept of normal-tension glaucoma is bankrupt. PC-OAG happens across a spectrum of IOPs—albeit lower overall than the IOPs seen with isolated-nasal-step glaucoma patients.
• More disc hemorrhages. As shown in the same chart, the frequency of disc hemorrhages in paracentral open-angle glaucoma patients is much higher than it is in the isolated nasal step patients. In fact, I’d speculate that sooner or later, all patients with paracentral open-angle glaucoma will be discovered to have a disc hemorrhage.
• A different genetic signature. A study we published in 2013 found that patients with PC-OAG have some genetic markers that are significantly different from those of patients with isolated peripheral visual field loss.2 Specific gene variants in the genomic regions between the caveolin 1 and caveolin 2 genes are more strongly associated with paracentral open-angle glaucoma than with patients who have primary open-angle glaucoma and isolated peripheral visual field loss.
We do
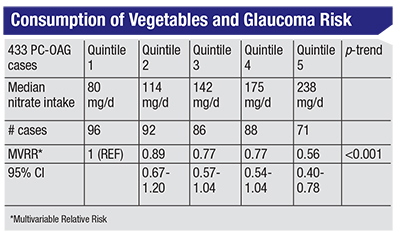 |
| A 2016 study found that the greatest consumption of nitrate from vegatables (238 mg/d) was associated with a 44-percent reduced risk of PC-OAG compared to those who consumed the least (80 mg/d).4 |
Managing PC-OAG
When we’re treating a patient with glaucomatous damage that’s attacking the center of vision, two strategies may be especially effective.
• Suggest increasing the dietary intake of nitrate-rich vegetables. Given that NO is almost certainty a factor in this subtype of glaucoma, it occurred to me and several of my colleagues that something as simple as dietary consumption of foods high in nitrates—such as vegetables—might favorably modify the risk of POAG. (Patients often ask if there is something they can do to alter the risk of disease progression, and a dietary change is a good option to suggest.)
We looked at this because of something called the nitrate-nitrite-NO pathway, which is a source of exogenous NO that’s well-known in the cardiovascular literature. When we consume vegetable nitrate, commensal bacteria in the mouth con-vert it to nitrite, which is very stable in our blood and can undergo either enzymatic or non-enzymatic conversion to NO. It’s a simple and straightforward way to deliver NO.
To see whether this would really have an impact, we conducted a study, published in 2016, of the association between dietary nitrate intake and primary open-angle glaucoma in the Nurses’ Health Study and the Health Professionals Follow-up Study.4 Many of the participants in those clinical trials were followed for more than 25 years. As you can see in the chart above, participants who consumed a median of about 238 mg/day of nitrate from vegetables had a 44-percent reduced risk of primary open-angle glaucoma compared to those who consumed the smallest quantity of nitrates from vegetables, about 80 mg/day. This supports the idea that NO can help to prevent this subtype of primary open-angle glaucoma.
To further confirm that NO signaling was associated with PC-OAG in particular, we separated those subjects with paracentral damage from those with peripheral visual field loss. Even though subjects with paracentral loss made up only about 25 percent of POAG cases, they were responsible for most of the association. (In subjects with peripheral visual field loss we found no statistical association with vegetable consumption.)
• Aim for a lower-than-usual target pressure. One other thing that I’ve noted in my two decades of man-aging patients with paracentral open-angle glaucoma: They require a lower target pressure than other glaucoma patients. For example, regardless of central corneal thickness, I’ve seen these patients progress at pressures lower than 16; that means 16 mmHg may be too high a pressure to help these patients. Ironically, another characteristic of this group of patients is that it can be very challenging to lower the IOP to the level required to stabilize their disease.
African-derived OAG
Another group of glaucoma patients with several unique characteristics is those of African descent. I refer to their group as having African-derived open-angle glaucoma, or AD-OAG. Among the characteristics associated with this category are:
• Early onset. For example, a 32-year-old African-American patient recently came to see me. He had a positive family history of glaucoma. Notably, he had lost most of his vision in the right eye some time ago; he had been told that he was essentially blind in that eye at an exam with another physician eight years earlier, at the age of 24. For whatever reason, his lost vision didn’t seem to faze him, and he didn’t pursue the matter.
When I examined him, he was unable to see the large E on the eye chart with his right eye, but was seeing 20/20 with the left. His untreated IOPs were 24 mmHg in the right eye and 18 in the left. His corneas were on the thin side, but a slit lamp examination didn’t reveal any sort of secondary glaucoma. On gonioscopy, his angles were open. Scans revealed that the right optic nerve was severely cupped and pale, and the visual field was essentially extinguished. His left eye showed some cupping, but a full visual field.
The fact that his vision was severely compromised by the time he was 24 means his glaucoma must have started when he was a teenager. His untreated IOPs were not extraordinarily high, although
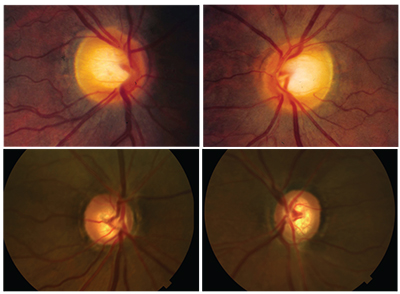 |
| AD-OAG is associated with early disease onset and reduced risk of disc hemorrhage. Top: 34-year-old patient. Tmax: 21 mmHg OU; CCT: 485 µm OU. Bottom: her 12-year-old daughter. Tmax: 12 mmHg; CCT 540 µm; visual fields normal. |
Another example: The images above show the discs of a 34-year-old woman of African descent and her 12-year-old daughter. The girl has normal pressures, corneas and visual fields; for this reason, the cupping seen in her discs might be construed as “physiologic.” However, her mother has moderate-stage primary open-angle glaucoma with thin corneas and IOPs not much higher than her daughter’s. You can see that she has erosion of both the inferior and superior neuro-retinal rims of both optic nerves. The odds are good that the cupping seen in her daughter’s optic nerves is a sign of oncoming glaucoma.
Another indicator of this early onset phenomenon can be found in a study I co-authored with Richard Parrish II, MD, and Richard Lee, MD, in which they screened an Afro-Carib-bean population living just south of Miami.5 Their data revealed something very striking, but not surprising to me: Almost 21 percent of people between the ages of 20 and 40 had either an IOP ≥24 or a cup-to-disc ratio ≥0.7, in at least one eye. Normally, we worry about older patients developing glaucoma; this data demonstrates that younger people of African descent can indeed develop glaucoma.
• Reduced risk of disc hemorrhage. We know that, generally speaking, disc hemorrhages are a very important independent risk factor for POAG. That was demonstrated by three very well-done studies using multivariate models: the Ocular Hypertension Treatment Study; the Early Manifest Glaucoma Trial; and the Collaborative Normal Tension Glaucoma Treatment Study. After controlling for IOP, disc hemorrhage was either associated with conversion from ocular hypertension to primary open-angle glaucoma, or with disease progression (in people who had mani-fest open-angle glaucoma).
In contrast, the African Descent and Glaucoma Evaluation Study (ADAGES) examined close to 10,000 disc photos from people who were of African or Caucasian ancestry, both with and without glaucoma. The data showed that people of African descent had an 80-percent reduced risk of having a disc hemorrhage. In other words, even though African heritage is a risk factor for glaucoma and glaucoma blindness, and disc hemorrhages are known to be an important factor for disease progression, people of African ancestry seem to develop disc hemorrhages rather infrequently. At this point, we don’t know the reason.
Managing AD-OAG
Given this, what can we do differ-ently when managing a patient of African descent?
• Remember that this type of disease starts early. As you can surmise from the two case histories cited earlier, many of these individuals may be going blind because doctors tend to assume glaucoma isn’t likely to be a problem until later in life. These younger individuals are simply not on our radar screen. So when faced with a young patient of African descent we should assume he or she might, in fact, have early glaucoma.
• Don’t assume that the absence of disc hemorrhages indicates that the eye is healthy. These eyes, even when diseased, tend to have fewer disc hemorrhages than you might find in the eyes of individuals with a different subtype of glaucoma.
• Educate these patients about the nature of the disease, and offer to examine other young family members. When we see relatively young patients of African lineage who have significant disease, we need to ask about their children, because this
 |
| Evidence suggests that estrogen levels may be associated with development and progression of glaucoma, as seen in this 61-year-old female patient. |
Estrogen-deficiency OAG
When seeing a female patient, we don’t often focus on estrogen-related milestones in life, or related aspects of the patient’s medical history. As a result, we may be missing a key glaucoma-related factor. Consider a 61-year-old Caucasian female patient I recently saw. She became a glaucoma suspect because of enlarged cups starting in the year 2000 when she was 47. She was status post hysterectomy; she’d had pre-eclampsia at age 30 and bilateral oophorectomy at age 53. In 2013 she noticed that the superior visual field in her right eye had collapsed.
When I examined her, she had concluded that her gynecological history had something to do with her vision loss. Her maximum IOP was 21 mmHg, central corneal thickness was 540 µm, and current IOPs on maximal medical therapy were 15 mmHg OD and 14 mmHg OS. Looking at her visual fields the right eye has a superior paracentral scotoma; the left eye has a superior nasal step. Was this just another case of primary open-angle glaucoma? Or was she right in believing that her gynecological history had something to do with her eyes doing poorly?
It turns out there’s a lot of evidence in the literature suggesting that the presence or relative absence of estrogen is associated with the development and progression of glaucoma. For example: Retinal ganglion cells have receptors for estrogen;6 optic nerve structure varies with the menstrual cycle;7 intraocular pressure decreases during pregnancy;8 postmenopausal hormones lower IOP;9,10 and retinal sensitivity on visu-al fields varies with the menstrual cycle.11 Furthermore, increased risk of POAG is associated with later menarche;12 oral contraceptive use;12,13 early menopause;14 and early oopho-rectomy.15
At the same time, events that extend estrogen exposure in a woman’s life, such as later menopause or post-menopausal hormone use, are associated with reduced risk of open-angle glaucoma.16-18 It’s also been shown that estrogen eye drops protect against retinal ganglion cell dropout in a rat model of glaucoma.19-21 In short, we have both clinical and population-based evidence that there may be a link between female reproductive health and POAG.
You may note that the damage seen in my patient’s visual fields is reminiscent of the damage caused in patients with PC-OAG. The right eye has a superior paracentral scotoma, and the left eye has a superior nasal step. This makes sense, because estrogen is a strong upregulator of NO signaling, a fact that’s well-demonstrated in the endocrine literature. So it’s not surprising there would be some overlap between estrogen deficiency open-angle glaucoma and PC-OAG.
So: How can we better help these patients? The simple answer is to be aware that this aspect of our female patients’ medical history may be very relevant to their potential for developing glaucoma or experiencing progression. Beyond that, it’s premature to suggest any alteration of hormonal status based upon this connection; there are far too many ramifications associated with altering hormone levels, including possibly causing cancer. Even estrogen-based eye drops may have systemic effects, so being aware of the connection and this part of our patients’ medical history may be the most we can afford to do—for now.
Practical Ramifications
Primary open-angle glaucoma is a remarkably heterogeneous dis-ease. There are probably many candidate secondary mechanisms at work, including factors such as impaired NO signaling. I believe that thinking of glaucoma as consisting of subcategories like the ones I’ve described here—as well as other categories that may become evident in the future—will make it easier for us to help our patients by helping us catch early glaucoma that we might otherwise have missed, and letting us offer treatments that are more precisely directed at the kind of glaucoma the patient is exhibiting. There is some overlap between PC-OAG, AD-OAG and ED-OAG, but they tend to have different ages of onset, different IOP profiles, different structural optic nerve features and different genomic biomarkers.
Making distinctions like this is also important for future research. If we lump all of these types of glaucoma together and just call them primary open-angle glaucoma, we’re missing an incredible opportunity to learn about each of them. Effects associated with one subtype in a clinical trial could easily disappear into the overall numbers, leaving us with less understanding of the disease than when we started.
Here are a few clinical strategies that will help you make the most of what we already know:
• In PC-OAG cases, don’t settle for a pressure of 16 mmHg. These patients need a lower pressure than that to prevent progression.
• Suggest eating more leafy green vegetables, especially if the patient has PC-OAG. We have some pretty convincing cohort evidence that eating a diet high in leafy green vegetables could potentially help prevent the onset of open-angle glaucoma, especially if the patient has PC-OAG. The literature has also demonstrated that this is good for our cardiovascular health.
• When treating patients of African descent, make sure they understand that their young relatives need to be checked for the disease. You’ll be surprised how of-ten you’ll find signs of the disease in young family members.
• Remember that “physiologic cupping” patients may be future glaucoma patients. When you encounter a patient who has a glaucoma-like disc but a full visual field—a patient you might assume has physiologic cupping—it’s very reasonable to examine that individual once a year. We now know that there’s a genetic predisposition to larger cups, and some of those genes are also associated with POAG.
• Take note of very high IOPs. The majority of POAG patients don’t have IOPs greater than 35 mmHg. For example, consider the Barbados Eye Study. This study analyzed the IOPs of Afro-Caribbean patients newly converted to primary open-angle glaucoma. The data showed that the majority of glaucoma damage happened at pressures between 18 and 24 mmHg.22 Like the African-derived patients we discussed earlier, these POAG patients typically don’t have pressures of 40 mmHg.
At the practical level, this means that when you do see a pressure of 40 mmHg, the patient might have primary open-angle glaucoma, but you need to think about other secondary causes of elevated pressure such as steroid exposure or secondary glaucoma. Do a more careful slit lamp exam. Make sure the patient doesn’t have exfoliation and isn’t using steroids for some reason. (People using a steroid cream on their skin seldom realize that it might have an effect on their eyes.)
• Be wary of patients with rapid-onset glaucoma. No matter what type of glaucoma a patient may have, rapid visual field loss is rare. It does happen, but when you encounter a case like this you should always be looking for secondary causes such as steroid use or eye rubbing. Consider obtaining diurnal IOP curves, because their IOP could be much higher at other times of day. Also, some of these patients may have occult neuro-ophthalmological disease and require neuro-imaging. In short, your antenna should go up when you see a patient who progresses very rapidly in a short period of time. REVIEW
Dr. Pasquale is a professor of ophthalmology at Harvard Medical School, director of the Glaucoma Service at Mass Eye and Ear Infirmary and co-director of Harvard’s Glaucoma Center of Excellence.
1. Park SC, De Moraes CG, et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology 2011;118:9:1782-9.
2. Loomis SJ, Kang JH, et al. Association of CAV1/CAV2 genomic variants with primary open-angle glaucoma overall and by gender and pattern of visual field loss. Ophthalmology 2014;121:2:
508-16.
3. Lei Y, Zhang X, Song M, et al. Aqueous humor outflow physiology in NOS3 knockout mice. Invest Ophthalmol Vis Sci. 2015 Jul;56(8):4891-8.
4. Kang JH, Willett WC, et al. Association of dietary nitrate intake with primary open-angle glaucoma: A prospective analysis from the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016;134:3:294-303.
5. Bokman CL, Pasquale LR, et al. Glaucoma screening in the Haitian Afro-Caribbean population of South Florida. PLoS One 2014;30;9:12:e115942.
6. Munaut C, Lambert V, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol 2001;85:7:877-82.
7. Akar ME, Taskin O, Yucel I, Akar Y. The effect of the menstrual cycle on optic nerve head analysis in healthy women. Acta Ophthalmol Scand 2004;82:6:741-5.
8. Phillips CI, Gore SM. Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol 1985;69:2:117-9.
9. Tint NL, Alexander P, et al. Hormone therapy and intraocular pressure in nonglaucomatous eyes. Menopause 2010;17:1:
157-60.
10. Vajaranant TS, Maki PM, Pasquale LR, et al. Effects of hormone therapy on intraocular pressure: The Women’s Health Initiative-Sight Exam Study. Am J Ophthalmol 2016;165:115-24.
11. Yucel I, Akar ME, et al. Effect of the menstrual cycle on standard achromatic and blue-on-yellow visual field analysis of women with migraine. Can J Ophthalmol 2005;40:1:51-7.
12. Wang YE, Kakigi C, et al. Oral contraceptive use and prevalence of self-reported glaucoma or ocular hypertension in the United States. Ophthalmology 2016;123:4:729-36.
13. Pasquale LR, Kang JH. Female reproductive factors and primary open-angle glaucoma in the Nurses’ Health Study. Eye (Lond). 2011;25:5:633-41.
14. Hulsman CA, Westendorp IC, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemiol 2001;154:2:138-44.
15. Vajaranant TS, Grossardt BR, et al. Risk of glaucoma after early bilateral oophorectomy. Menopause 2014;21:4:391-8.
16. Pasquale LR, Rosner BA, et al. Attributes of female reproductive aging and their relation to primary open-angle glaucoma: a prospective study. J Glaucoma 2007;16:7:598-605.
17. Newman-Casey PA, Talwar N, et al. The potential association between postmenopausal hormone use and primary open-angle glaucoma. JAMA Ophthalmol 2014;132:3:298-303.
18. Na KS, Jee DH, et al. The ocular benefits of estrogen replacement therapy: A population-based study in post-menopausal Korean women. PLoS One 2014;9:9:e106473.
19. Prokai-Tatrai K, Xin H, et al. 17b-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm 2013;10:8:3253-61.
20. Russo R, Cavaliere F, et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res 2008;173:583-90.
21. Zhou X, Li F, Ge J, et al. Retinal ganglion cell protection by 17-beta-estradiol in a mouse model of inherited glaucoma. Dev Neurobiol 2007;67:5:603-16.
22. Leske MC, Connell AM, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol 2001;119:1:89-95.



