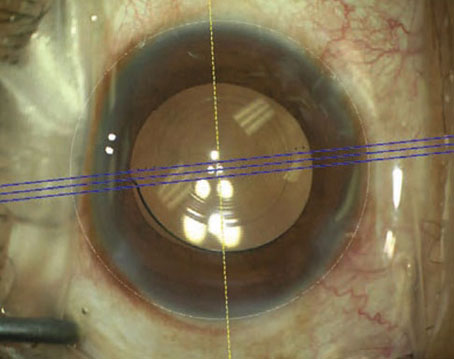
The very first multifocal intraocular lens to be used in the United States was the AMO Array, approved by the FDA about two decades ago. Since then, numerous companies have been designing and developing unique models of these presbyopia-correcting lenses—and even a lens-correcting laser—to broaden the number of options available to patients with varying expectations and desired outcomes. These lenses may offer presbyopes a more dynamic range of vision, and even spectacle independence in some cases.
Much of the new tech aims to reduce the negative visual effects that can occur in patients following implantation of a diffractive IOL, the most common of these being reduced contrast sensitivity and dysphotopsias (i.e., nighttime glare, halos and starbursts). Besides tried-and-true multifocal IOLs, there are several other presbyopia-correcting IOL technologies attempting to make their way into ophthalmic practice in the coming years. Here, we’ll discuss where each of these presbyopic IOLs is in the pipeline, the mechanisms behind each design and what preliminary trials have demonstrated so far about their potential visual and clinical outcomes.
Trifocal IOLs
Two years ago, in the summer of 2019, the PanOptix IOL (Alcon) became the first and only trifocal IOL to date to receive FDA approval. Its advent provided a new solution for patients in search of a premium IOL with the ability to give some improvement of vision at near, intermediate and distance. Specifically, the PanOptix lens distributes half of the light for distance and 25 percent each for intermediate and near vision. Other trifocal IOLs being developed have varying percentages of light distribution.
In terms of downsides, trifocal lenses can have a negative impact on night vision as a result of splitting the light in three ways to accommodate each of the three focal points. Patients can also experience halos, starbursts and glare, as well as some reduced contrast around objects. Depending on a patient’s unique goals, lifestyle and occupation, any of the following trifocal IOLs currently making their way through the pipeline may offer an expanded range of vision.
• EnVista trifocal (Bausch + Lomb). B+L is currently conducting trials on this addition to its enVista family of IOLs. The enVista trifocal will include the same features as other enVista lenses, with the added bonus of a more continuous range of vision from distance to near. The single-piece hydrophobic acrylic is currently under investigational use in the United States, and B+L is getting ready to conduct its Phase III clinical trial.
The enVista trifocal IOL, along with the other enVista IOLs, is made with what B+L calls the “TruSight” optic design, consisting of a material 25 times harder than a traditional hydrophobic acrylic lens, which the company says increases its resistance to scratches and abrasions.1 The company adds that the lens haptics, dubbed “AccuSet,” were also created to be durable and stable. B+L says the design provides lens stability and a 300 percent greater radial compression force than traditional hydrophobic acrylic.1
“We’re currently enrolling for the enVista trifocal IOL Phase III clinical trial,” says Chuck Hess, vice president and general manager of U.S. Surgical, Bausch + Lomb. “This multicenter, randomized study will include more than 500 subjects undergoing bilateral cataract surgery. Subjects will receive either enVista trifocal IOLs or enVista monofocal IOLs. Investigators will determine efficacy endpoints after six months and safety endpoints after 12 months based on post-surgical observation.” Mr. Hess notes that the study is intended to support a Pre-Market Approval application filing with the FDA.
As far as potential candidates and outcomes for the enVista trifocal IOL, Mr. Hess explains that while it’s a bit early to talk specifics, “Clinical trial results will allow [B+L] to determine the specific attributes of the investigational lens,” and mentions that the company is developing a toric version as well. In addition to this trifocal IOL for correcting presbyopia, Mr. Hess shares that B+L is also working on several extended-depth-of-focus IOL technologies currently being researched and developed.
“The more IOLs that are available, the greater the opportunity for physicians to meet the specific needs of each individual patient,” says Mr. Hess.
 |
• AT LISA trifocal (Zeiss). Another presbyopia-correcting IOL that could be making its way into your practice is the AT LISA trifocal IOL, which is already approved for use in a number of countries around the globe. (“LISA” is an acronym that summarizes the concept behind the lens: Light distributed asymmetrically, Independence from pupil size, SMP technology (for a smooth lens surface) and Aberration-correcting optimized aspheric optic.) 2
The AT LISA trifocal is made of a hydrophilic acrylic material with hydrophobic surface properties and has an optic diameter of 6 mm and a total diameter of 11 mm. The power ranges from zero to +32 D in 0.5-D increments. It requires a small 1.8-mm incision for its insertion using the company’s BlueMICS 180 injector.
A study of 227 eyes of 114 patients who underwent bilateral implantation of the AT LISA trifocal found that after 12 months, outcomes for binocular uncorrected distance, intermediate and near visual acuity were ≤0.3 logMAR (20/40) in 99, 98.1 and 91.4 percent of eyes, respectively, and patients achieved normal contrast sensitivity at six months postop.2 As for patient satisfaction, the study reports that 12 months after surgery, 93.3 percent, 89.4 percent and 84.6 percent of patients were satisfied or very satisfied with their distance, intermediate and near vision, respectively.2
To reduce the most commonly reported negative effect of diffractive IOLs, dysphotopsias, Zeiss designed the lens to distribute light asymmetrically between distant (65 percent) and near focus (35 percent), which the company says also results in improved intermediate vision.3
• RayOne trifocal. Rayner recently developed its own hydrophilic acrylic IOL for presbyopia called the RayOne trifocal, a lens with 16 rings in a 4.5-mm diffractive zone with a total optic diameter of 6.25 mm. The power range for this lens is zero to +30 D in increments of 0.5 D. The company says that the design of the RayOne trifocal is aimed at decreasing the incidence of visual disturbances and night vision problems. Rayner says it’s also designed to be less dependent on pupil size or lighting conditions in order to improve vision and accommodation around the clock for near, intermediate and distance.
The company says the lens’ patented diffractive step technology can transmit 89 percent of light to the retina with a 3-mm pupil. Roughly half the light is allocated for distance; the remaining half is divided between near and intermediate vision.
A study of 15 patients (30 eyes) comparing the performance of this lens to that of the FineVision POD F IOL (PhysIOL, Liège, Belgium) found that the RayOne trifocal demonstrated better refractive accuracy and milder issues with depth perception in patients than the latter, although other photic phenomena were not statistically different between groups.4 Both IOLs were able to achieve positive visual outcomes in all patients. At three months postop, the study reported the mean monocular distance visual acuity to be 0.03 ± 0.11 (RayOne trifocal) and 0.04 ± 0.08 (FineVision POD F) logMAR (20/21 and 20/22, respectively); distance-corrected intermediate visual acuity, 0.05 ± 0.13 and 0.05 ± 0.10 logMAR (both around 20/22); and distance-corrected near visual acuity, 0.02 ± 0.12 and 0.03 ± 0.11 logMAR (both around 20/22).4
Clinical trials are still ongoing for this lens.
AcuFocus IC-8
 |
| AcuFocus IC-8 |
The AcuFocus IC-8, possibly the closest in the pipeline to entering your practice, is a clear, aspheric monofocal lens that uses wavefront-filtering and small aperture technology to disrupt peripheral light rays and allow central focused light to hit the retina. In short, it creates a pinhole effect through its unique design of a ring with a small hole in the center. AcuFocus says that the IC-8 is designed to offer patients a continuous range of vision with fewer bothersome visual effects that typically accompany diffractive IOLs, such as problems with night vision and photic phenomena.
Just last month, on December 7th, the IC-8 received a letter from the FDA indicating it had completed its review of the IC-8® IOL Premarket Approval (PMA) Application and determined that the PMA substantially meets the requirements of the Food Drug and Cosmetic Act. The letter communicates that the FDA believes it can approve the application following successful completion of the pre-approval inspections at the company’s manufacturing facilities in the U.S. and internationally.5
The 6-mm single-piece hydrophobic acrylic lens has a 2.23-mm-diameter opaque mask with a 1.36-mm central aperture. Using a unique injector system, insertion requires a 3.5-mm incision. In clinical trials investigating its ability to correct presbyopia, the company reports that, “Six months after implantation with a small aperture IOL in one eye, 99 percent, 95 percent and 79 percent of patients with presbyopia achieved 20/32 or better binocular uncorrected distance, intermediate and near visual acuity, respectively.”6
“The advantage of the design of this lens is that it allows for non-focused or irregular light rays to be filtered out by the pinhole,” says Mark H. Blecher, MD, co-director of the Cataract and Primary Eye Care Service at Wills Eye Hospital in Philadelphia and the chief medical editor of Review. “As a result, refractive error, including hyperopia, myopia and astigmatism, are minimized, and aberrant light from an irregular cornea is minimized. Ultimately, it improves the depth of focus of the eye and decreases the number of higher-order aberrations because the irregular light rays aren’t allowed in. Depending upon your refractive target, it can give you good distance vision and it can give you depth of focus for intermediate vision.”
Dr. Blecher says the desired target refraction may determine whether this lens will be a good choice for a given patient. “The effect is not powerful enough to provide a patient with full reading vision most of the time, but its success depends on what you’re targeting the refraction to be,” Dr. Blecher explains. “If you pick a myopic target, the patient will get more near vision, and the pinhole will still give them good distance vision.” He notes that the recommended target for this lens is -0.75 D.
Y. Ralph Chu, MD, founder and medical director of Chu Vision Institute in Bloomington, Minnesota, is eager to be able to offer this new accommodative lens to various types of patients. “The IC-8 is exciting because it helps increase depth of focus through an aperture effect; there’s really no other lens like that available right now in the market,” says Dr. Chu. “Not only can it be used as a presbyopia-correcting IOL, but it could also be used potentially to help patients with irregular astigmatism or irregular corneas. I can picture a lot of patients with corneal disease—not just from previous refractive surgery, but from medical conditions like keratoconus, injuries to the eye where the pupil is damaged or irregular astigmatism caused by a corneal scar—possibly benefiting from the IC-8 and the improved quality of vision.”
It’s possible the IC-8 might be approved in 2022.
Perfect Lens
Now for something a little different in the pipeline: rather than a physical accommodative lens, this alternative method of presbyopia correction, the Perfect Lens (Perfect Lens, LLC), involves a femtosecond laser that can change the refractive power of a previously implanted lens. The innovative technology aims to offer a less-invasive solution to post-cataract surgery patients or those who received a presbyopia-correcting IOL previously and end up needing or desiring a different refractive power months or years down the line.
The Perfect Lens is currently only in the early phase of human trials outside the United States, but according to literature published so far, the results seem promising. The lens uses refractive index shape technology that involves adding water to designated areas of the pre-implanted lens, therefore changing its refractive characteristics, according to the company.7 The company also says that laboratory studies show that “RIS technology can be used to change an existing IOL power of up to 3.6 D within 23 seconds while keeping a good modulation transfer function.” The results also showed the technology is capable of switching a lens back and forth from multifocal to monofocal.8
“It’s a pretty cool idea to have a truly customizable lens where, for the first time, it’s been shown in the laboratory that you can take a monofocal lens and turn it into a multifocal lens and then turn it back into a monofocal lens without loss of quality of vision,” says Dr. Chu. “In a way, it could be like an eraser for a surgeon. For example, a patient would be able to try multifocality, and if they can’t tolerate the side effects at night or are unhappy with the outcome for whatever reason, then theoretically, according to laboratory results up to this stage, you could actually erase the multifocality with this technology and the patient could go back to monofocal lenses.”
The advantages of reversibility and customization are the two major elements of Perfect Lens that set it apart from other presbyopic IOLs in the pipeline, notes Dr. Chu. “You can customize the prescription, astigmatism and presbyopia correction all with that same laser, but more importantly, you can reverse the correction, which is something that no other lens or IOL technology can do at this stage.”
Juvene
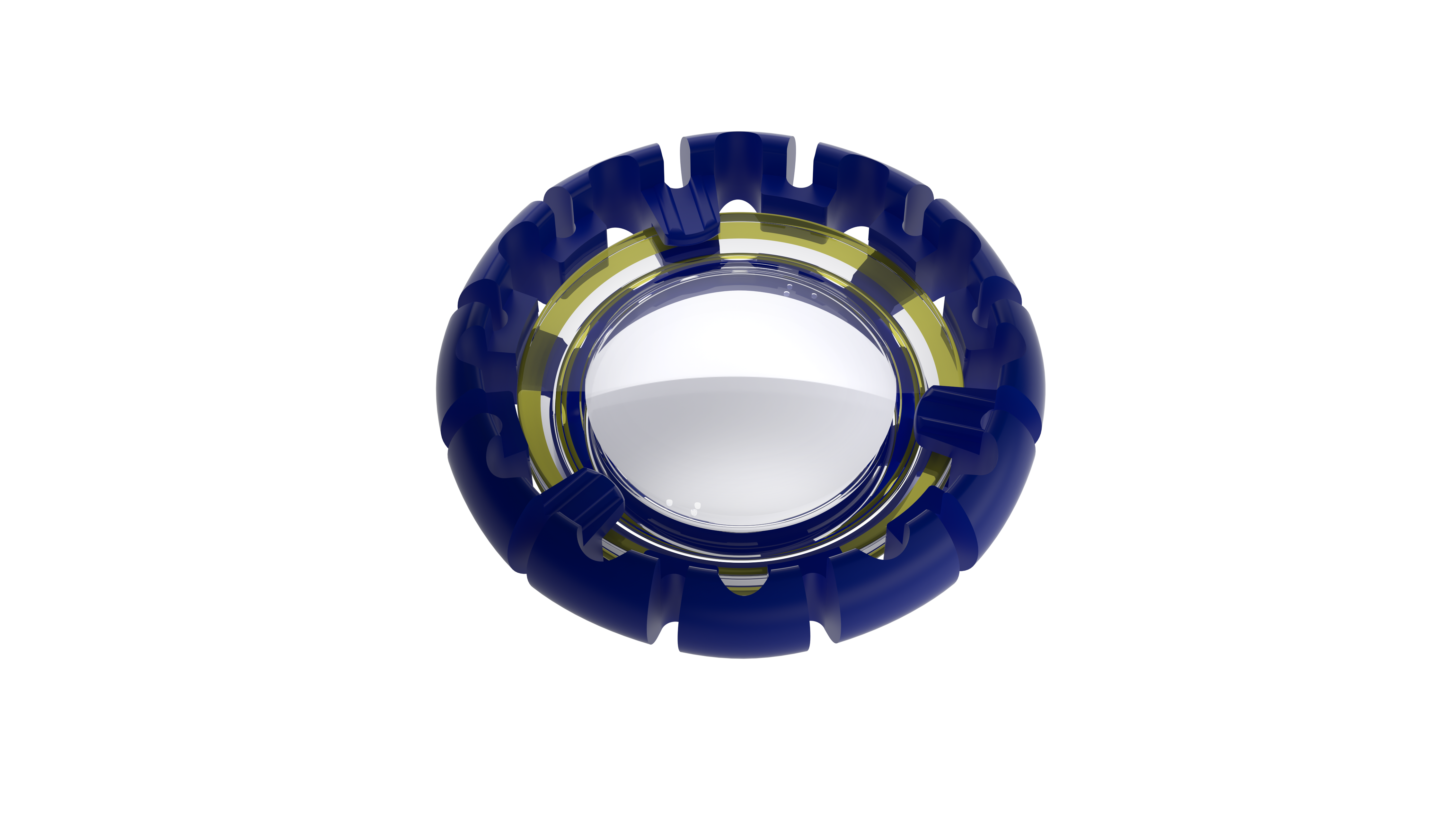 |
| Juvene IOL (LensGen) |
The world’s first modular fluid optic IOL, Juvene (LensGen, Irvine, California), is a premium lens for presbyopia in the pipeline that tries to help patients achieve true accommodation. The two-part lens is designed to mimic the effect of the natural crystalline lens, and it can be inserted through a 3-mm incision. It consists of a base lens that fills the capsular bag and a curvature-changing liquid silicone lens that fits into the base lens and allows for more seamless accommodation, which could offer patients a continuous dynamic range of vision from distance to near. Also, presbyopes bothered by visual dysphotopsias caused by IOLs with concentric rings may benefit from a non-ring, fluid-optic IOL such as the Juvene.
“The base lens keeps the capsular bag distended and hopefully clear, as well as provides a big chunk of the focusing ability that you need post-cataract,” says Dr. Blecher. “Then, you have a second smaller lens component, the fluid lens, that you put in, which moves in the eye and changes shape to give you that accommodation on demand.”
He brings up the point that when placing multi-piece IOLs, the rate of success in achieving targeted visual outcomes may decrease. “When you put these lenses in, you want the patient to have clear distance vision, so you have to hit your refractive target, and then the accommodative effect will provide the near. But, when you’re working with a two-piece IOL like Juvene, the predictability of achieving that is a little less.” Another downside of a multi-piece premium IOL, besides increasing the complexity of surgery, could be the likely higher cost.
Auspicious trial results were published by LensGen in 2019 from the company’s Grail study, which involved 54 eyes implanted with the IOL by multiple surgeons (14 patients underwent bilateral implantation). In September 2019, Sumit Garg, MD, presented the following conclusions from the study based on one to six months of patient follow-up data: 100 percent of eyes achieved spectacle independence, 100 percent achieved 20/25 vision at distance and intermediate and 91 percent achieved 20/32 at near. The company also reported that no patients in the study experienced glare, halo or other dysphotopsia. Eric D. Donnenfeld, MD, presented the updated study’s results on patient refractive outcomes at one year postop at the 2020 virtual American Academy of Ophthalmlology meeting. He reported that patients achieved a mean monocular CDVA, DCIVA and DCNVA of 20/20, 20/25+ and 20/32-2, respectively.10
The Juvene IOL received approval for an Investigational Device Exemption from the FDA at the end of November 2021, meaning the company may soon begin conducting human trials to study the safety and effectiveness of the lens.11
FluidVision
The FluidVision lens (Alcon/PowerVision) is another IOL that uses fluid inside the lens to provide what the company calls “true accommodation.” The IOL is a hollow, acrylic single-piece lens that’s placed in the capsular bag. The lens is filled with silicone fluid that moves in response to contraction or relaxation of the ciliary muscles. When the eye is in its natural accommodative state, a drop of fluid moves from the haptics to the center of the IOL, causing the IOL to slightly inflate and allow near vision. As the eye moves to its disaccommodative state, the lens deflates by squeezing the liquid back over to the haptics, yielding distance vision.
The FluidVision IOL has an optic diameter of 6 mm and a total diameter of 10 mm, making it larger than many other IOL models and therefore requires a wider incision. The company says the newest version of the lens, the FluidVision 20/20, can be implanted through a 3.5-mm wound using the PowerJect injector system; however, studies are under way investigating a model that will decrease the incision size required for insertion.
This lens is currently still in its investigational stage.
JelliSee
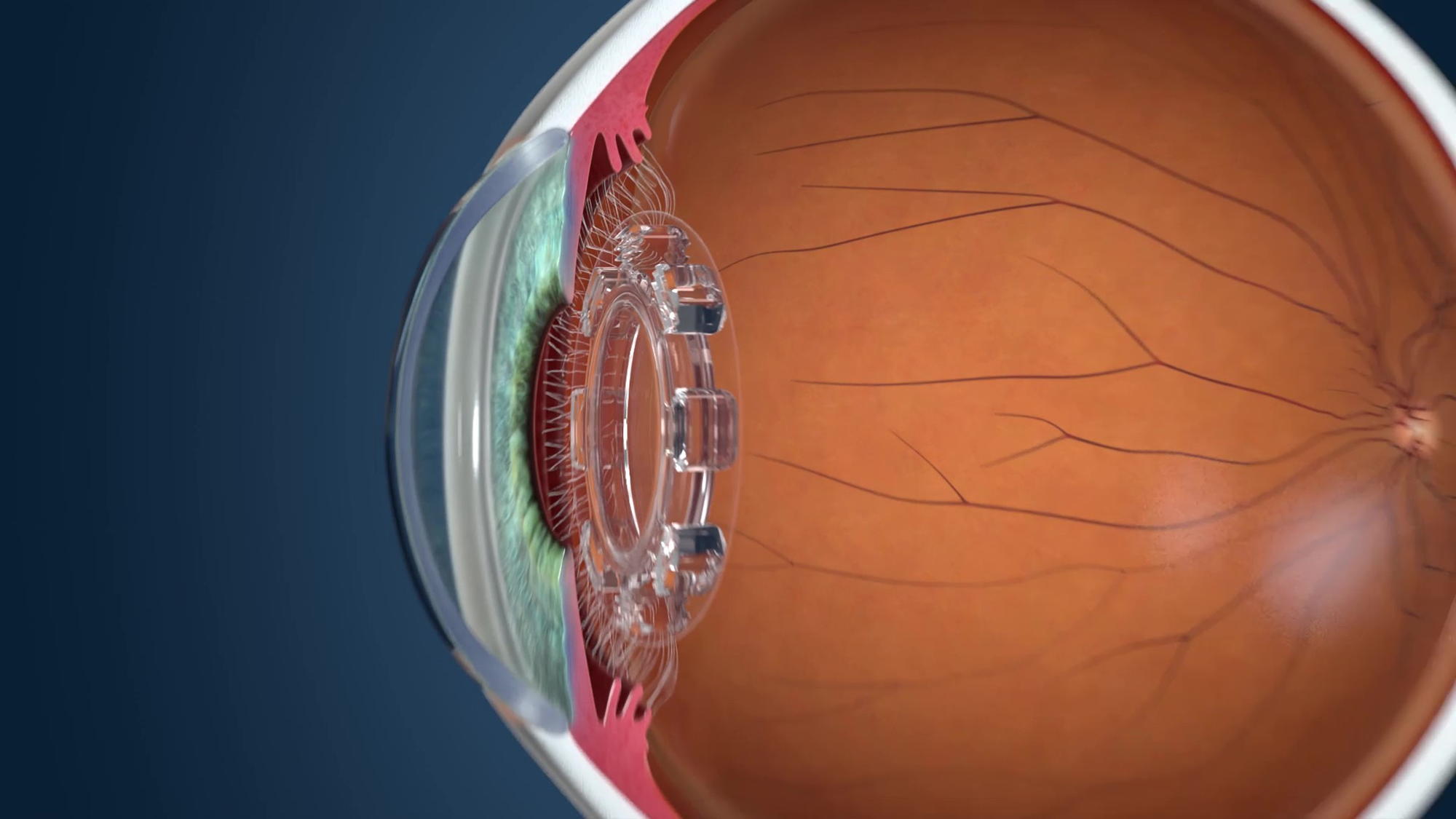 |
| JelliSee accommodating IOL |
The newest addition to the growing list of presbyopic IOLs in the pipeline, the JelliSee (JelliSee Ophthalmics) accommodating IOL is a monofocal lens finishing up its preclinical stage that aims to provide presbyopes and astigmats with true accommodation.
The company says that, as the eye shifts its focus between near and distant targets, the flexible design of JelliSee permits it to react to natural forces of the ciliary muscle using technology similar to that used in the FluidVision and Juvene IOLs. As the muscle relaxes, the lens will flatten and increase in diameter in reaction to the force the zonules exert onto the lens capsule, enabling the focus to change from near to far.
One thing that sets this IOL apart from others, according to developers, is its ability to allow focus to shift from very near to very far with just a fraction of a millimeter of diameter change. Preliminary results from bench studies performed by the company have demonstrated that the lens can achieve 6 D or greater accommodation with less than 0.2 mm of diameter change, and that it provides smooth and immediate transitions across all ranges (near, distance and intermediate) with minimal dysphotopsias and minimal effect on contrast sensitivity. 12
“We have initial confirmation of this accommodation in preclinical work and are progressing to further clinical trials in the near future,” says the company’s CEO and president, Jim Ellis, MD, who reports that human studies are scheduled to begin this spring (2022). “The proof of concept has been validated with bench testing as well as in vivo.”
Dr. Ellis adds, “Anyone with presbyopia would be a candidate for this IOL.” He notes that the design of the JelliSee makes it a suitable lens for patients with ocular surface, retinal or macular disease who aren’t typically ideal candidates for posterior chamber IOLs.
“Because the JelliSee IOL is a shape-changing monofocal lens, just like our natural lens when we were young, these contraindications don’t apply,” explains Dr. Ellis. He also notes that since the IOL is independent of retained capsular elasticity, its functionality should also not be affected by capsular fibrosis.
Dr. Blecher was a paid investigator for Acufocus in the IC-8 IOL study. Dr. Chu was a principal investigator for AcuFocus IC-8 and is on the medical advisory board for Perfect Lens.
1. Bausch + Lomb. enVista IOL. Updated 2021. Accessed December 1, 2021. https://www.bausch.com/ecp/our-products/cataract-surgery/lens-systems/envista-iol.
2. Aggarwal RK. Clinical outcomes of different multifocal IOLs. XXV Congress of the ESCRS 2007; Stockholm, Sweden.
3. Piovella M, Colonval S, Kapp A, Reiter J, Van Cauwenberge F, Alfonso J. Patient outcomes following implantation with a trifocal toric IOL: Twelve-month prospective multicentre study. Eye (Lond). 2019;33:1:144-153.
4. Ferreira TB, Ribeiro FJ. Prospective comparison of clinical performance and subjective outcomes between two diffractive trifocal intraocular lenses in bilateral cataract surgery. J Refract Surg. 2019;35:7:418-425.
5. AcuFocus Receives Approvable Letter from the U.S. FDA for the IC-8 Small Aperture Intraocular Lens. AcuFocus. Accessed December 1, 2021. https://acufocus.com/press/acufocus-receives-approvable-letter-from-the-u-s-fda-for-the-ic-8-small-aperture-intraocular-lens.
6. Dick HB, Piovella M, Vukich J, et al. Prospective multicenter trial of a small aperture intraocular lens in cataract surgery. J Cataract Refract Surg. 2017;43:956-68.
7. Perfect Lens. Updated 2021. Accessed December 1, 2021. http://www.perfectlens.com/index.htm.
8. Results. Perfect Lens. Updated 2021. Accessed December 1, 2021. http://www.perfectlens.com/results.htm.
9. Garg, S. Visual performance of eyes in the Grail Study. LensGen. Oral presentation at: International Society of Presbyopia; September 13, 2019; Paris, France. https://presbyopia-international.com/wp-content/uploads/2019/12/Garg_Grail-Study.pdf.
10. Accommodating IOLs: 1-Year Outcomes. AAO. Updated January 22, 2021. Accessed December 16, 2021. https://www.aao.org/interview/accomodating-iol?fbclid=IwAR0mRrrPh39b31D2d4Xy3JLaAZ1CKg2eEEmKnqnrh0UQTYD2f3AjwIbCu_w.
11. LensGen. LensGen receives IDE approval from the U.S. FDA to begin clinical study of the Juvene presbyopia-correcting intraocular lens. PR Newswire. November 29, 2021. Accessed December 1, 2021. https://www.prnewswire.com/news-releases/lensgen-receives-ide-approval-from-the-us-fda-to-begin-clinical-study-of-the-juvene-presbyopia-correcting-intraocular-lens-301432541.html.
12. Technology. JelliSee Ophthalmics, Inc. Updated 2021. https://www.jellisee.com/technology.html.