In this world, nothing can be said to be certain, except death and taxes—and presbyopia. This inevitable loss of near accommodation affects more than 120 million individuals in the United States and more than two billion globally. Presbyopia treatments have typically included reading glasses, contact lenses, refractive procedures and presbyopia-correcting implants, but there are several pharmacologic candidates in the pipeline now, as well as an FDA-approved drop.
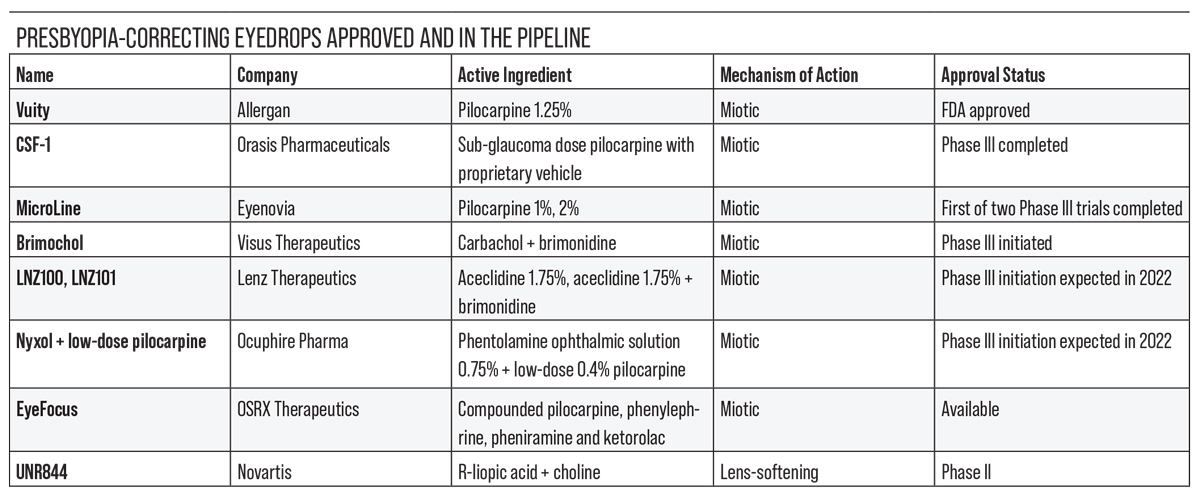
The current presbyopia-correcting drops in development either constrict the pupil to improve near vision or reduce lens stiffening to improve accommodation. In this article, we’ll review some of the drops in the pipeline, their mechanisms of action and how they’ve been performing in clinical trials.
The Pharmacologic Option
Y. Ralph Chu, MD, founder and medical director of Chu Vision Institute and Chu Surgery Center in Bloomington, Minnesota, says that presbyopia-correcting drops are bringing some patients to the clinic who haven’t seen an eye-care provider in a long time. “It starts a conversation about the patient’s refractive options,” he says. “Some of these patients became surgical candidates because of their pre-existing refractive error or other medical indication, such as a dysfunctional lens or early cataract.”
“The first compound to come out is Vuity, and it’s done a phenomenal job of establishing the presbyopia eyedrop category,” says Steven J. Dell, MD, of Dell Laser Consultants in Austin. “But I think it’s pretty clear that this is an enormous new category of pharmaceutical usage, and there will be room for more than one player in this space.”
“Different presbyopia drops’ mechanisms of delivery will appeal to different subsets of patients,” agrees David Wirta, MD, of Newport Bay Surgery Center and Hoag Physician Partners in Newport Beach, California. “I think it’s reasonable for more than one version of pilocarpine [as well as other active ingredients] to be available to patients.”
Now that an eyedrop is available, what do clinicians consider when offering presbyopia-correcting eyedrops to their patients? “You know, I think it’s the reverse,” says Dr. Chu. “These drops are getting a lot of play in direct-to-consumer marketing, and patients are asking us about them first.
“We spend a lot of time educating staff to prepare for patient questions,” he says. “The presbyopia classification scale published in 2021 in Ophthalmology and Therapy by Marguerite McDonald, MD, and colleagues, classifies presbyopia into mild, moderate or advanced severity. It’s been our roadmap for educating our staff and patients about what to expect from the drop.
“Patients want a drop to get them out of reading glasses, so they’re wondering if they’re candidates for the drop,” he continues. “We start our process by going over patients’ prescriptions and making sure they undergo a complete eye exam to determine eye health, especially retinal health. Then, we start educating and setting expectations.”
Dr. Chu says that setting expectations begins by understanding what the patient’s pre-drop reading vision is without glasses. “If a patient starts with vision between J5 to J8, they typically tend to be very happy with the drop’s results, regardless of their age. That’s a good starting point for practices beginning to offer Vuity.”
Dr. Chu notes that not all patients will achieve a level of reading vision with Vuity that enables them to be completely free of reading glasses, but some have noticed improvements in intermediate vision. “I have a patient who started with poor Jaeger vision, around J10, and I told them that they’ll still probably need reading glasses, but they may notice better computer vision,” he says. “Two weeks later, they called back and said, ‘You’re exactly right. I still need reading glasses, but I can see my kids play hockey without my glasses, and I can use my computer.’ They were happy with the outcome of the drop. This is a good example of the importance of educating patients and setting the right expectations.”
 |
| Click image to enlarge. |
In the Vanguard
Allergan’s Vuity (pilocarpine hydrochloride ophthalmic solution 1.25%) was approved in October 2021. It’s indicated for once-a-day dosing in adults with mild to moderate presbyopia.
Vuity is made with Allergan’s pHast technology. This novel buffering solution allows the pilocarpine to be stored acidic in the bottle (pH range 3.5 to 5.5) but to adjust rapidly to physiologic pH upon contact with the ocular surface. Though its clinical significance is unknown, Dr. Chu, a principal investigator of Vuity, says it may improve pilocarpine’s bioavailability and efficacy.
“Pilocarpine acts by two mechanisms,” explains Dr. Chu. “It constricts the pupil in a dynamic way and has a small effect on the ciliary body muscles. Studies have shown that reducing the pupil size to about 40 to 50 percent of the pre-drop level helps improve depth of focus without affecting distance vision. That’s the dynamic pupil modulation process.
“Secondly, pilocarpine stimulates the ciliary body to constrict, which can also create some improved reading vision,” he says. “This effect is more prominent among younger presbyopes (around 40 years of age) since these patients may still have some ciliary body muscle effect.”
Allergan says Vuity offers improved near and intermediate vision without compromising distance vision. In GEMINI-1 (n=323)1 and GEMINI-2 (n=427),2 Vuity demonstrated significant near vision improvement under mesopic conditions out to six hours, and under intermediate lighting conditions, the effect lasted out to 10 hours.
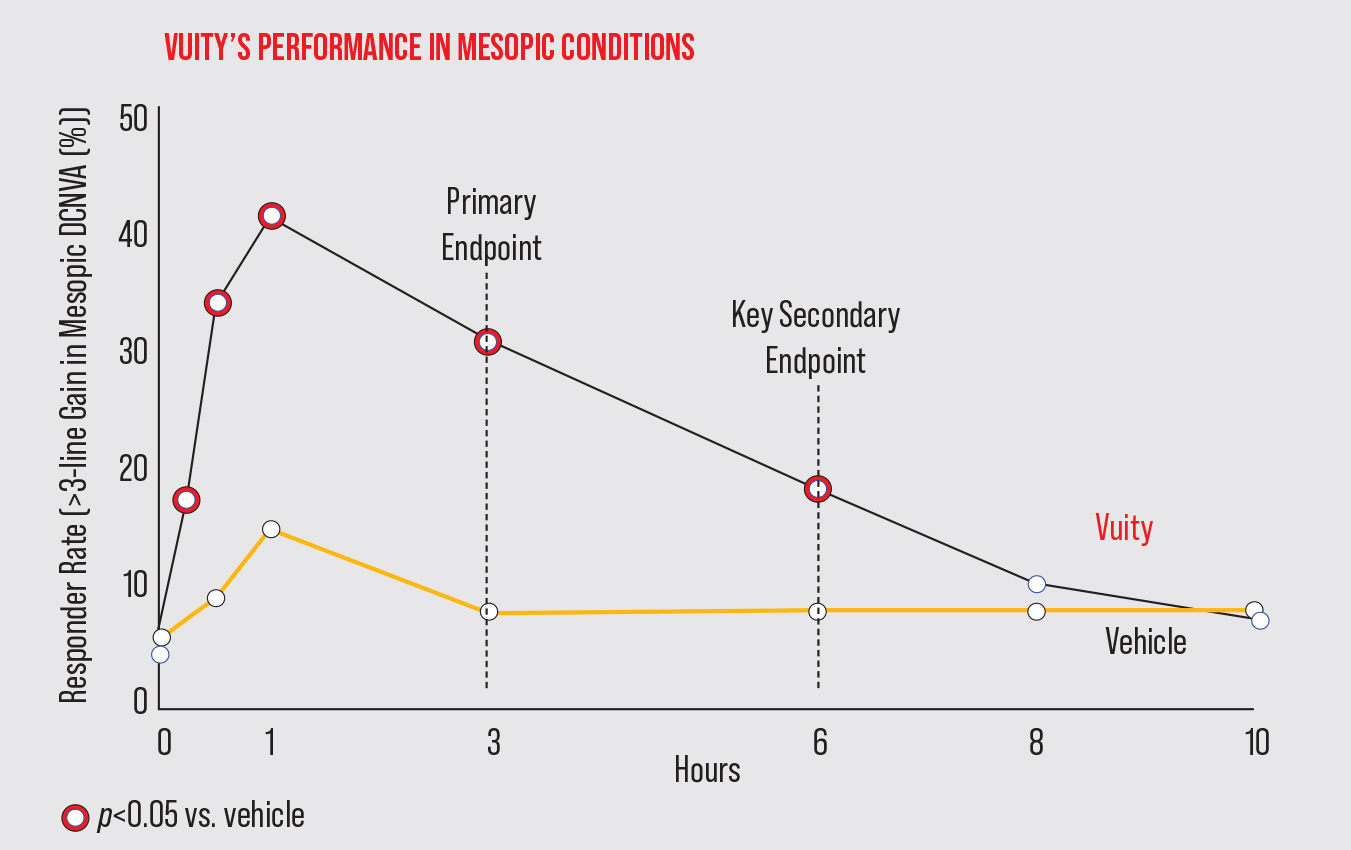 |
| In the Phase III GEMINI-1 study, Vuity met its primary and secondary efficacy endpoints of a ≥3-line gain at three and six hours, respectively. There was a significant difference between Vuity and vehicle from 15 minutes to six hours post administration. (Adapted from Waring G, et al. Presented on July 25, 2021 at the ASCRS Meeting.) Click image to enlarge. |
In the primary efficacy results among the intent-to-treat population, significantly more study participants gained ≥3 lines of mesopic distance-corrected near visual acuity without losing more than one line of corrected distance visual acuity at day 30, hour three, compared with vehicle (GEMINI-1: 31 percent Vuity vs. 8 percent vehicle [p<0.01]; GEMINI-2: 26 percent Vuity vs. 11 percent vehicle [p<0.01]).
Additionally, a post-hoc analysis reported that one-third of subjects randomized to Vuity achieved 20/20 DCNVA without losing more than five letters of CDVA on day 30 at hour one (GEMINI-1: 33.5 percent Vuity vs. 7.8 percent vehicle; GEMINI-2: 33.2 percent Vuity vs. 13.6 percent vehicle). Allergan notes that because this finding wasn’t part of a pre-specified endpoint and could represent chance findings, these data should be interpreted with caution.
There were no reported retinal detachments with Vuity use in the two clinical trials, though rare cases of retinal detachment have been reported with the use of other miotics3 in susceptible individuals.
Dr. Chu reports that Vuity was well-tolerated by patients in both clinical trials. “Mild side effects included headache, blurry vision and some redness of the eye,” he says. “About 10 to 15 percent of patients reported mild headache—mainly transient brow ache.
“These headaches are manageable in the real world, and they typically get better with a few days of Vuity use,” he points out. “To ward off headache, patients can take an OTC painkiller about half an hour before instilling the drop. I think it’s important to set expectations with patients, so they understand why they’re getting a headache. In a way, it shows that the drop is working. Additionally, we found that treating the ocular surface with artificial tears in the real world helps to reduce the amount of dryness on the ocular surface so patients can overcome these mild irritation symptoms more quickly.”
Next in Line: Low-dose Pilocarpine
 |
| Vuity uses a novel buffering solution that enables the pilocarpine to be stored acidic in the bottle. It adjusts to physiologic pH upon contact with the ocular surface. Photo: Vuity |
The next presbyopia-correcting eyedrop in line for FDA approval is likely Orasis Pharmaceuticals’ CSF-1, a preservative-free, low-dose pilocarpine (0.4%) hydrochloride solution with a proprietary vehicle. Like other pilocarpine formulations, it creates a pinhole effect to increase depth of field and the ability to focus on near objects. Orasis says CSF-1 doesn’t compromise distance or night vision.
CSF-1 was tested in two Phase III clinical trials, NEAR-1 (n=309)4 and NEAR-2 (n=304),5 which were launched in October 2020. The company announced positive topline results in April 2022. “In both trials, CSF-1 met its primary and secondary endpoints, which were the number of patients improving three lines or more at three hours, and no loss of one line or more in distance visual acuity,” says Dr. Wirta, a NEAR-2 trial investigator. “CSF-1 was statistically superior to placebo.”
In the trials, one drop of CSF-1 or placebo was administered bilaterally twice daily for approximately two weeks to 613 presbyopic patients (aged 45 to 64). Participants attended four study visits: screening, and days one (baseline), eight and 15. Pooled results demonstrated that 40 percent and 50 percent of participants gained three or more lines one hour after the first dose and one hour after the second dose, respectively (p<0.0001). A statistically significant three-line improvement was achieved at all time points on days one and 15. On day 15, the >3-line improvement in DCNVA was seen at 20 minutes and for up to eight hours after the first dose.6
“CSF-1 demonstrated fewer of pilocarpine’s most common side effects, namely short-term headache or brow ache (6.8 percent),” Dr. Wirta notes. “There was also a low incidence of stinging upon instillation among participants (5.8 percent), which is a common pilocarpine side effect.”
A Novel Delivery System
Eyenovia is developing an investigational, proprietary pilocarpine 2% formulation for presbyopia treatment called MicroLine. In May of 2021, the company announced positive results from its first Phase III study, VISION-1 (NCT04657172, n=84),7 which met its primary outcome measure, the proportion of treated subjects who gained three or more lines in DCNVA versus placebo in low light conditions at two hours post-treatment.
“We tested two different concentrations of pilocarpine: 1% and 2%,” says Dr. Wirta, a study investigator. “The 2% concentration was significantly more effective at improving near vision compared with placebo (OR: 7.7; p<0.05).” This was determined by measuring the improvement in high-contrast binocular DCNVA measured in low-light conditions two hours after treatment.
MicroLine is notable for its unique form of delivery to the ocular surface. The drug comes pre-packaged in Eyenovia’s Optejet microdosing spray dispenser, which delivers the solution to the ocular surface in a directional mist, rather than in an eyedrop. Eyenovia describes the Optejet and its microdose array print technology as a miniature inkjet printer that coats the ocular surface with microdroplets akin to pixels.
“This method administers about one-fifth the volume of solution compared with a traditional eyedropper,” Dr. Wirta says. “Less excess medication in the eye may improve tolerability and reduce the occurrence of undesirable side effects. It also cuts down on wasted medication that might otherwise spill out of the patient’s eye.
“Patients receiving MicroLine in the Optejet see an effect last from three to six hours,” Dr. Wirta continues. “In VISION-1, we saw very few of pilocarpine’s usual side effects, such as headache and dim vision. Less than three percent of the MicroLine-treated patients reported brow or headache.” He says this may be due to the Optejet delivery method.
 |
| The Optejet device delivers the MicroLine solution to the ocular surface in a directional mist, rather than in an eyedrop. Using microdroplets avoids waste and may lessen certain side effects associated with overdelivery of medication to the ocular surface, Eyenovia says. MicroLine is currently in Phase III trials. |
The VISION-2 trial,8 a double-masked, placebo-controlled, cross-over superiority trial is testing MicroLine 2% using the Optejet spray dispenser in approximately 140 presbyopic subjects. The primary endpoint is improvement in high-contrast binocular DCNVA measured in low-light conditions two hours after treatment. Topline data from VISION-2 is expected in Q2 of 2022.
Dynamic Duo
In March 2022, Visus announced the initiation of its Phase III pivotal trials (BRIO-I and BRIO-II) for Brimochol-PF, a presbyopia-correcting eyedrop that’s a combination of carbachol 2.75% and brimonidine tartrate 0.1%. Brimochol-PF relies on a small-aperture optic approach to increase depth of field and depth of focus for presbyopic patients.
Cathleen McCabe, MD, of The Eye Associates in Sarasota, Florida, says that the combination drop performed well in the Phase II VIVID studies, which completed in November 2021. Two formulations were tested: one with benzalkonium chloride and the other without, as well as Visus’ Carbachol-PF (carbachol 2.75%). “We wanted to know if there was any important contribution from the BAK,” says Dr. McCabe.
Though BAK is often a culprit behind adverse side effects in topical drops that contain it, BAK can actually facilitate drug penetration and improve efficacy by disrupting the epithelial junctions.
As Brimochol-PF’s name suggests, the study found no increased efficacy or detriment associated with the preservative-free or BAK-containing formulations, so the Phase III trial will focus on the preservative-free formulation of Brimochol vs. Carbachol-PF.
Dr. McCabe says that in the VIVID study, the combination therapy reduced pupil size more than Carbachol-PF alone. “It reached the pupil-size goal of 2 to 3 mm and had a duration of action that seems like it’ll increase depth of field and near vision for about six to eight hours,” she says. “Looking at the data and the number of responders with the FDA-set parameter of a three-line improvement in near vision without a loss of a line of distance vision, the number of responders was higher in the Brimochol group from the three-hour time point on, and that persisted. In fact, the deviation between Carbachol-PF alone and Brimochol increased as the duration of the test increased.”
Dr. McCabe points out that the VIVID study included many older patients (age range: 45 to 80). “This might be the oldest population of enrolled patients in presbyopia drop studies,” she says. “They could be phakic or pseudophakic. Brimochol still showed significant improvement in near vision without loss of vision in these older patients.
“Brimochol also exhibited very few side effects,” she notes. “The only side effects reported in 5 percent or more subjects were transient burning or stinging upon instillation and headache. Headaches were mild and transient.”
A patient-reported outcomes portion of the VIVID study found that, in a Marketscope survey of 1,000 U.S. customers who were presbyopic but who hadn’t received any presbyopia drops, the average desired duration for presbyopia-correcting eyedrops was 8.1 hours. “That’s pretty much the sweet spot that Brimochol reached,” Dr. McCabe points out.
Interestingly, some patients in the VIVID study demonstrated improvement in distance vision. “The lack of a reduction in distance vision shows that the effect isn’t simply myopia,” she says. “On average, patients saw better at distance than they did prior to instillation.”
Dr. McCabe says Brimochol-PF’s first Phase III readouts may be available in Q4 of this year.
A New Mechanism of Action
You may have seen this company’s active ingredient, aceclidine, connected with the name PRX-100 or LiquidVision (aceclidine + tropicamide) under a different manufacturer’s name, Presbyopia Therapies, last year. Based on positive Phase II trials for PRX-100 that supported aceclidine’s use for presbyopia in the aceclidine-only arm,9 Lenz Therapeutics, is now developing preservative-free aceclidine 1.75% (LNZ100) and aceclidine 1.75% + brimonidine (LNZ101).
Lenz says both drugs use the company’s proprietary vehicle matrix to improve bioavailability.10 The company considers the LNZ100 formula to be the optimal concentration for pinhole effect, while noting that LNZ101 may have the potential for increased duration and the added benefit of eye whitening.
Dr. Dell says that one of aceclidine’s features distinguishing it from pilocarpine or carbachol is its significant decoupling of the miotic effect and the stimulation of the ciliary muscle, with accompanying myopic shift. “Aceclidine targets different muscarinic receptors than pilocarpine,” he says. “With pilocarpine and carbachol, there tends to be a shift in the myopic direction that accompanies miosis. Patients tend to get a brow ache with ciliary muscle contraction. With aceclidine, there’s an absence of ciliary muscle activity. One theoretical benefit of that is that patients may experience a lower risk of retinal tears.”
Aceclidine met its primary endpoints for near vision improvement in the Phase II studies. The company reports that 81 percent of individuals gained at least two lines of vision and 53 percent gained at least three lines within 30 minutes. Half of the study participants maintained a two-line improvement and 22 percent maintained a three-line improvement for at least seven hours, with pupil diameters ranging from 1.5 mm at one hour to 2 mm at seven hours vs. 5.1-mm diameter at pre-dose. Dr. Dell says they found this pupil diameter to be the sweet spot.
Additionally, compared to placebo, aceclidine resulted in no change in best-corrected normal-light DVA (p≥0.99) or best-corrected low-luminance DVA (p≥0.25). The most common side effects were mild discomfort on instillation. “There were no serious adverse events in the clinical trials,” Dr. Dell points out.
“While this drug has never been used in the United States before, it has a historical pattern of use in Europe, where it’s been used in more than 400 million doses beginning in the 1970s for glaucoma,” he says. “The problem with it as a glaucoma drug was that it was never as good as pilocarpine for pressure control. There’s a lot of safety data for the higher concentrations of aceclidine used in Europe—it was even used at a q.i.d. dosage.”
The company is preparing for a pivotal Phase III trial in 2022 to compare LNZ100 with LNZ101.
Long-lasting Miosis
Nyxol (phentolamine ophthalmic solution 0.75%) is Ocuphire Pharma’s multitasking, preservative-free eyedrop candidate. When combined with a drop of low-dose pilocarpine (0.4%), it may treat presbyopia. Alone, it’s being studied for reversal of mydriasis and for night vision disturbances.
Nyxol alone has demonstrated moderate pupil-diameter reduction and improvement in near visual acuity, but the addition of low-dose pilocarpine may allow the formula to achieve the pinhole effect to improve depth of focus and near reading vision, says Ocuphire.
“Phentolamine is a non-selective alpha-1 and alpha-2 adrenergic agonist that works on the sympathetic nervous system to prevent dilation and enable the pupil to constrict for improved depth of focus,” explains Dr. Chu, who is a clinical investigator for Ocuphire. “Phentolamine avoids some of the potential perceived risks of [higher doses of] pilocarpine such as retinal detachment. Its duration of action is undetermined, but it seems as though it could be a once-a-day drop.”
Ocuphire completed its Phase II VEGA-1 trial11 for Nyxol + low-dose pilocarpine in May 2021, and the Phase III trial is expected to launch this year. In VEGA-1 (n=150), Nyxol + low-dose pilocarpine achieved three lines or more of binocular near vision in photopic conditions in one hour in 61 percent of treated patients compared with 28 percent of the placebo group (p=0.003). The combination demonstrated a rapid, 30-minute onset with improved near vision lasting at least six hours. Pupil-diameter reduction lasted at least 18 hours. Ocuphire points out that Nyxol alone has a duration of longer than 24 hours.12
No serious adverse events were reported in the study. There were no reported headaches, brow aches or blurry vision, though fewer than 5 percent of subjects reported mild, transient eye redness.13 The company plans to advance to Phase III registration trials this year.
Compounded Therapies
Compounded therapies may be more cost-effective for patients, and some compounding pharmacies even mail the eyedrops directly to patients’ homes, making this a convenient option. Here’s one option available in the United States and the drop that inspired it:
• EyeFocus (OSRX Pharmaceuticals). EyeFocus is a compounded combination drop from Missoula, Montana-based OSRX Pharmaceuticals. This presbyopia-correcting drop was developed with and licensed from Luis Felipe Vejarano, MD, creator of FOV Tears, another presbyopia-correcting drop (see below). Both EyeFocus and FOV Tears produce similar effects, inducing miosis and improving accommodation.
Eyefocus is available from OSRX Pharmaceuticals for $100 per 9-ml bottle in two different concentrations:14 EyeFocus contains pilocarpine HCl (0.302%), phenylephrine HCl (0.624%), pheniramine (0.0772%) and ketorolac (0.01%); and EyeFocus+ contains pilocarpine HCL (0.604%), phenylephrine HCL (0.624%), pheniramine (0.0772%) and ketorolac (0.01%).
As a compounded drop, EyeFocus hasn’t gone through the FDA regulatory pathway, and neither concentration is available to be prescribed. It’s expected to produce results similar to FOV Tears, which is currently available in Colombia.
• FOV Tears. This drop is instilled bilaterally, twice daily. It consists of pilocarpine (0.247%), phenylephrine (0.78%), polyethyleneglycol (0.09%), nepafenac (0.023%), pheniramine (0.034%) and naphazoline (0.003%).
In a 2019 clinical study,15 the authors explained their rationale for including each component: “Pilocarpine produces ciliary body contraction and stimulates accommodation; it also induces miosis, which increases the depth of focus. Naphazoline intensifies the relaxing effect of pilocarpine on the dilator pupillae. Nepafenac, pheniramine and phenylephrine counteract ciliary muscle spasm, hyperemia and excessive pupil constriction induced by pilocarpine. Polyethyleneglycol lubricates the eye and stops the burning sensation caused by all the other drugs, leveling the pH.”
Results from the study (117 eyes treated with FOV Tears, mean age 50.2; baseline UNVA 0.35 logMAR) indicated that the drop was effective. FOV Tears improved near vision by one or more lines in 92.3 percent of patients two hours after instillation, demonstrating a significant mean improvement of 0.18 lines (p=0.000). Nine patients saw no improvement in UNVA, but no patients lost any lines of vision. Headache was reported as a side effect in 11.9 percent of patients. Additionally, younger patients (aged 41 to 50) gained more lines of near vision than older patients (aged 51 to 65), which was likely because the younger patients had more residual accommodative function.
A Lens-softening Agent
As the crystalline lens hardens with age, it loses some of its accommodative range and ability to focus on near objects. Lens-softening agents, such as Novartis’ investigational presbyopia-correcting drop UNR844 (formerly under research by Encore Vision as EV06), may restore some degree of lens flexibility, allowing for better accommodation.
UNR844 is a prodrug that uses lipoic acid choline ester 1.5% to reduce the disulfide bonds in the crystalline lens. When UNR844 penetrates the cornea, it’s metabolized into choline and R-lipoic acid. The lipoic acid is broken down by the lens into its active form, dihydrolipoic acid or DHLA. This DHLA is thought to be responsible for reducing the disulfide bonds and increasing the lens’ elasticity. A Phase I/II study reported a five-letter improvement in DCNVA vs. placebo, with benefits lasting up to seven months.
In December 2019, Novartis completed a Phase II study of UNR844 that included presbyopic patients (aged 45 to 65) randomized to receive either 1.5% UNR844 ophthalmic solution (as a chloride salt) (n=40) or placebo drops (n=38), dosed binocularly, twice daily for three months.16 The primary endpoint was change in binocular DCNVA from baseline at three months in patients aged 45 to 55.
The study found no significant difference in mean change in DCNVA between UNR844 and placebo (difference: 1.6 letters, p=0.1832), and the primary endpoint wasn’t met.17 The researchers performed a post-hoc non-parametric analysis due to high variability in both groups’ measured DCNVA. Greater variability was seen in the placebo arm. This analysis found that the mean difference between UNR844 and placebo was four letters (p=0.0924), which the authors wrote was closer to the Phase I/II study results. They noted no clinically meaningful differences in either ocular or systemic side effects between the two arms.
UNR844 was also tested in a 2021 company-sponsored safety and preliminary efficacy randomized controlled trial, with results supporting its continued development.18 A total of 75 patients were randomized 2:1 to UNR844 or placebo. Subjects were dosed unilaterally twice a day on days one to seven in the nondominant eye. Bilateral dosing occurred from days eight to 91. UNR844 was well-tolerated, produced no safety concerns and induced no clinically relevant changes in BCDVA, pupil size or IOP. The authors noted that DCNVA improved in the study eye in the treatment group compared with placebo (mean change: -0.159 logMAR (20/14) vs. -0.079 logMAR (20/16); p=0.007). Bilateral DCNVA improved, with 53.1 percent of UNR844 patients gaining ≥10 letters compared with 21.7 percent of placebo patients. DCNVA improvements persisted five and seven months after UNR844 dosing completion.
A Phase II study involving 237 participants testing different concentrations of UNR844 is estimated to be completed in March 2023.19 The primary outcome measure is change from baseline in binocular DCNVA at three months.
This article has no commercial sponsorship.
Dr. Chu is a consultant for Allergan and Ocuphire Pharma. Dr. Wirta is a consultant for Eyenovia. He has received grant support from Eyenovia and Orasis for his role as a principal investigator in clinical studies. Dr. Dell is a consultant for and shareholder in Lenz Therapeutics. Dr. McCabe is a consultant for Visus.
1. Allergan. A Phase 3 multicenter, double-masked, randomized, vehicle-controlled, parallel-group study evaluating the safety and efficacy of AGN-190584 in participants with presbyopia. ClinicalTrials.gov identifier: NCT03804268. Updated December 28, 2021. Accessed May 13, 2022. https://clinicaltrials.gov/ct2/show/NCT03804268.
2. Allergan. A Phase 3, multicenter, double-masked, randomized, vehicle-controlled, parallel-group study evaluating the safety and efficacy of AGN-190584 in participants with presbyopia. ClinicalTrials.gov identifier: NCT03857542. Updated December 29, 2021. Accessed May 13, 2022. https://clinicaltrials.gov/ct2/show/NCT03857542.
3. Vuity FDA Full Prescribing Information. Warnings and precautions. Accessed May 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214028s000lbl.pdf.
4. Orasis. A multi-center, double-masked, vehicle-controlled, evaluation of the efficacy and safety of CSF-1 in temporary correction of presbyopia (the NEAR-1 Study: Near Eye-vision Acuity Restoration). ClinicalTrials.gov identifier: NCT04599933. Updated February 24, 2022. Accessed May 16, 2022. https://clinicaltrials.gov/ct2/show/NCT04599933.
5. Orasis. A multi-center, double-masked, vehicle-controlled, evaluation of the efficacy and safety of CSF-1 in temporary correction of presbyopia (the NEAR-2 Study: Near Eye-vision Acuity Restoration). ClinicalTrials.gov identifier: NCT04599972. Updated February 24, 2022. Accessed May 16, 2022. https://clinicaltrials.gov/ct2/show/NCT04599972.
6. Orasis Pharmaceuticals announces positive phase 3 topline results of novel eye drop candidate, CSF-1, for the treatment of presbyiopia. Orasis Pharmaceuticals Press Release. Accessed May 17, 2022. https://www.orasis-pharma.com/orasis-pharmaceuticals-announces-positive-phase-3-topline-results-of-novel-eye-drop-candidate-csf-1-for-the-treatment-of-presbyopia.
7. Eyenovia. A Phase 3 study of the safety and efficacy of 1% and 2% pilocarpine ophthalmic solutions administered with the Optejet Microdose Dispenser for temporary improvement of near vision in adults with presbyopia. ClinicalTrials.gov identifier: NCT04657172. Updated August 9, 2021. Accessed May 13, 2022. https://clinicaltrials.gov/ct2/show/NCT04657172.
8. Eyenovia. A Phase 3 study of the safety and efficacy of 2% pilocarpine ophthalmic spray administered with the Optejet Microdose Dispenser for temporary improvement of near vision in adults with presbyopia. ClinicalTrials.gov identifier: NCT05114486. Updated May 9, 2022. Accessed May 13, 2022. https://clinicaltrials.gov/ct2/show/NCT05114486.
9. Lenz Therapeutics. A single-center, double-masked evaluation of the efficacy and safety of PRX-100 in the treatment of early to moderate presbyopia. ClinicalTrials.gov identifier: NCT03201562. Updated May 14, 2021. Accessed May 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03201562.
10. Lenz Therapeutics. May 2022 Corporate Presentation. https://lenz-tx.com/wp-content/uploads/2022/04/LENZ-corporate-presentation-May-2022.pdf. Accessed April 29, 2022.
11. Ocuphire. Safety and efficacy of Nyxol (0.75% phentolamine ophthalmic solution) with pilocarpine eye drops in subjects with presbyopia. ClinicalTrials.gov identifier: NCT04675151. Updated November 15, 2021. Accessed May 17, 2022. https://clinicaltrials.gov/ct2/show/NCT04675151.
12. Nyxol eye drops. Phase 2 efficacy and safety data. Ocuphire. Accessed May 17, 2022. https://www.ocuphire.com/product-pipeline/nyxol.
13. Ocuphire’s VEGA-1 phase 2 trial in presbyopia meets primary and secondary endpoints. Ocuphire Pharma Press Release. Accessed May 17, 2022. https://www.ocuphire.com/news-media/press-releases/detail/344/ocuphires-vega-1-phase-2-trial-in-presbyopia-meets.
14. OSRX Pharmaceuticals. EyeFocus formulations: Presbyopia drops. Accessed May 18, 2022. https://www.osrxpharmaceuticals.com/sites/default/files/resource-files/OMNI_EyeFocus_Brochure_v3_0.pdf.
15. Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: A prospective, consecutive interventional non-comparative clinical study. Ophthalmol Ther 2019;8:1:31-39.
16. Novartis. A 3-month, randomized, placebo-controlled, double-masked, multi-center study to evaluate the safety and efficacy of topical ocular UNR844-Cl in subjects with presbyopia. ClinicalTrial.gov identifier: NCT03809611. Updated May 11, 2022. Accessed May 18, 2022. https://clinicaltrials.gov/ct2/show/NCT03809611.
17. Richdale K, Wirta D, Venkataraman S, et al. UNR844 ophthalmic solution for the topical treatment of presbyopia: Results of a Phase II randomised controlled trial. Scientific Program Abstract presented at the 2020 American Academy of Optometry Meeting. Accessed May 19, 2022. https://www.aaopt.org/detail/knowledge-base-article/papers-novel-pharmaceutical-treatments.
18. Korenfeld MS, Robertson SM, Stein JM, et al. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye (Lond.) 2021;35:12:3292-3301.
19. Novartis. A randomized, placebo-controlled, double-masked, multi-center, dose-ranging study to evaluate the safety, and efficacy of UNR844 in subjects with presbyopia. ClinicalTrials.gov identifier: NCT04806503. Updated May 17, 2022. Accessed May 18, 2022. https://clinicaltrials.gov/ct2/show/NCT04806503.