|
Plasma is a term used to describe certain types of ionized gases. It comes in many different forms, some of which make modern technologies like cellular telephones and plasma televisions possible. It can be generated in ways that produce heat (e.g., the YAG laser, welding arcs, lightning in a thunderstorm or the sun) and in ways that do not (e.g., plasma television, fluorescent bulbs and the aurora borealis). Medical devices may be designed to produce either thermal or non-thermal plasma, depending on the purpose of the device. When plasma comes into contact with tissue, depending on the nature of the plasma being generated, it can act as a blade, disintegrating tissue in its path; it can kill bacteria without causing structural tissue damage; or it can trigger apoptosis in cancer cells without harming healthy cells.
Here, three individuals working with these types of plasma applications discuss their work.
Attacking Tumors
Michael Keidar, professor of mechanical and aerospace engineering at the School of Engineering and Applied Science, professor of neurological surgery at the School of Medicine and Health Sciences, and director of the GW Institute for Nanotechnology at The George Washington University, has done extensive work over the past several years in the area of using plasma to cause selective destruction of cancer tumors. “I got interested in the biomedical application of plasma about seven years ago,” he explains. “Plasma is an ionized gas that is typically generated in high-temperature laboratory conditions. Recent progress in atmospheric plasmas, however, has led to the creation of cold atmospheric plasmas, or CAP, with ions at close to room temperature. Today, 20 years of intense research effort applying CAP to bioengineering has led to the foundation of a new field: plasma medicine. And the most recent research area in the field of plasma medicine is the application of CAP to cancer therapy.”
Recent studies have shown that CAP has the ability to induce cell death via apoptosis and cell cycle arrest, both in vitro and in vivo. While malignant tumors can become resistant to chemotherapy, they appear to have a specific vulnerability to treatments involving the production of reactive oxygen and nitrogen species. In vivo, CAP has been shown to inhibit tumor growth, decrease proliferation, reduce tumor size and induce apoptosis. Furthermore, it’s been shown to affect at least 20 different varieties of cancer (including breast, colon, lung, bladder, ovarian, skin, pancreatic and prostate).1-14
Perhaps most important, unlike conventional cancer therapies, CAP seems to selectively kill cancer cells. “Based on many results to date, CAP could potentially be used to treat retinoblastoma,” he notes. “Plasma can be applied directly to a tumor with a penetration of about 5 mm, and no thermal damage is associated with its use.”
|
Professor Keidar says that handheld CAP tools are already available, and his group is developing new ones. However, he believes the ones they’re developing are several years away from being clinically available.
Cutting Without Resistance
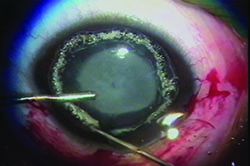
Although some uses for plasma technology are still in the investigative stages, at least one plasma tool has been available for several years: the Fugo blade, a handheld surgical instrument invented by Richard Fugo, MD, PhD, (Norristown, Pa.) that allows sharp, resistance-free cutting. The blade works by generating a cloud of plasma particles around a tiny filament at the end of a handpiece; the plasma dissolves the molecular bonds of any material it comes into contact with. As a result, the blade creates an incision sharper than an incision made with a diamond blade, and it can cut through thick, tough tissue easily. It also causes almost no bleeding if small vessels and capillaries are cut. Because of these characteristics, surgeons can perform operations that would be nearly impossible with a standard blade, causing little, if any, trauma to the eye.
Daljit Singh, MS, DSc, is the founder of the Daljit Singh Eye Hospital in Amritsar, India, and coauthor of the book Ocular Applications of the Fugo Blade. Dr. Singh (who has no financial interest in the Fugo blade) has used it for more than 15 years, pioneering numerous procedures that take advantage of the unique abilities of the instrument, including anterior capsulotomy, transciliary filtration and peripheral iridotomy (all approved by the U.S. Food and Drug Administration).
“The Fugo blade brings laser-like plasma to the fingertips of the surgeon,” explains Dr. Singh. “The plasma is created by focused electromagnetic waves generated by four rechargeable batteries; when in use, the plasma is visible as a throbbing yellow glow of light. When an activated tip touches tissue, the energy is transferred via the process of resonance. The tissue molecules become unstable and shatter, thus creating an incision path without burning or charring, and histology has confirmed the absence of collateral thermal damage. In fact, the Fugo blade incision is actually an ablation. The plasma also ablates any blood vessels in the incision path, resulting in a bloodless cut.”
Dr. Singh says the plasma blade is especially useful for creating an anterior capsulotomy. “It’s the best tool for creating a capsulotomy that I’ve found,” he says. “It successfully tackles any kind of capsule, at any age. Whether the capsule is normal, thick and scarred, or of unequal thickness, it cuts easily and the cut never runs out to the periphery. It’s quite different from older approaches because the surgeon perceives no tactile sensation; the cutting is resistance-free.”
|
Dr. Singh says he has also used the plasma blade to develop strabismus surgery techniques, for both weakening and strengthening, that maintain the insertion line by preserving the muscle strips at the margins. “This prevents the surgery from causing any misalignment,” he explains. “These strabismus techniques are bloodless and only take a short time to perform, and the postoperative course is inflammation-free, thanks to the peculiar cutting properties of the plasma blade. I’ve also been able to develop unique fornix and orbital approaches to levator and Müller muscle plication, for all grades of ptosis. I do this through three vertical fornix incisions made with the plasma blade. And because it ablates tissue, it takes only seconds to do punctoplasty or to clear blocked canaliculi.” Dr. Singh says that during the past 10 years, the retina surgeon at his eye hospital has successfully operated on three cases of Sturge-Weber syndrome caused by vascular tumors on the retina, using the Fugo blade. “All of these cases were refused by the best retina centers in India,” he notes.
“This is an very versatile surgical tool,” he concludes. “I believe many more eye surgeons around the world will eventually be using it.”
Plasma Disinfection
Another medical use for plasma is the sterilization of tissue, which can be done without causing structural damage using the right parameters. One surgeon working on making this a practical reality is David S. C. Pao, MD, who practices in Levittown, Pa. Early in his career, Dr. Pao invented and patented numerous ophthalmic instruments, including one of the first irrigation/aspiration machines for use in extracapsular cataract surgery; one of the first electroretinography instruments, designed to record the electrical signals generated by the retina and transmitted to the occipital cortex, which is still in use worldwide; and one of the first instruments used for coaxial bipolar cautery. One of the instruments he’s currently developing will use plasma to sterilize tissue, with the goal of helping to reduce the incidence of endophthalmitis.
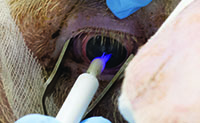
“This instrument will produce a non-thermal plasma between the probe and the tissue being treated, a space of 3 mm or less,” he explains. “It will ionize gases that are present in the air, primarily oxygen, nitrogen and hydrogen. The effect this has on the tissue will depend on the parameters used, which published reports show can range from antibacterial effects to enhancing tissue healing, as well as the destruction of melanoma cells through apoptosis. As a tool for sterilization, this could be used on cataract corneal wounds, retinal surgery scleral wounds or intravitreal conjunctival-scleral injection sites.”
Dr. Pao became interested in the potential for sterilizing tissue using non-thermal plasma after meeting professor Gregory Fridman at the A.J. Drexel Plasma Institute in Philadelphia. Professor Fridman was conducting plasma experiments with Staphylococcus aureus contamination of the spinal cord of New Zealand white rabbits. Wanting to see whether this could be applied to the eye, Dr. Pao began inoculating the rabbits’ corneas with the bacteria and then treating it using a prototype plasma device. This early work showed that simple treatment resulted in a two-log reduction in S. aureus from an initial concentration of ~106 CFU/mL.
|
Dr. Pao notes that there are many potential uses for a non-thermal plasma probe like this, beyond ocular surgery. “A device like this could be used to reduce contamination during medical procedures in rural areas where there are few medical resources,” he points out. “It could be used in combat zones to clean wounds. It could be used to sanitize hands in hospitals without risking creating bacterial resistance. Think of having a machine on the wall that you place your hands inside; it lights up for three seconds and sterilizes your hands. A device using this technology could decontaminate the walls, floors and ceilings of operating rooms as well as patient rooms in hospitals. Certainly it could be used to sterilize surgical equipment. It’s possible that it might even work on biological entities such as prions that are resistant to heat sterilization, although that remains to be proven.”
Because excess treatment can cause structural damage to the tissue, Dr. Pao is currently working with live rabbit and porcine eyes at the Drexel School of Medicine Animal Lab, studying the impact of different voltages, pulse durations and energy frequencies, to refine the ideal parameters for causing sterilization without tissue damage. (He credits Kristina Pao, MD, Justine Han, BS, and Ralph Eagle, MD, with major contributions to this work.) He’s also working toward making the device available to the medical community. “Right now, we’re meeting with a company that’s interested in commercializing this,” he says. “The final commercial unit should be similar to many of the devices we already use in cataract surgery; the prototype’s design is similar to the Alcon crescent blade. Hopefully this will become available within the next year or two.” REVIEW
1. Kim CH, Bahn JH, Lee SH, Kim GY, Jun SI, Lee K, Baek SJ. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol 2010;150:4:530-8.
2. Sensenig R, Kalghatgi S, et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann Biomed Eng 2011;39:2:674-87.
3. Keidar M, Walk R, Shashurin A, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer 2011, 25;105:9:1295-301.
4. Kim JY, Ballato J, Foy P, et al. Apoptosis of lung carcinoma cells induced by a flexible optical fiber-based cold microplasma. Biosens Bioelectron 2011;28:1:333-8.
5. Arndt S, Wacker E, Li YF, et al. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Exp Dermatol 2013;22:4:284-9.
6. Daeschlein G, Scholz S, Lutze S, et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp Dermatol 2013;22:9:582-6.
7. Utsumi F, Kajiyama H, Nakamura K, et al. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS One 2013;8:12:e81576.
8. Wang M, Holmes B, Cheng X, et al. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS One 2013, 11;8:9:e73741.
9. Köritzer J, Boxhammer V, Schäfer A et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS One 2013;8:5:e64498.
10. Walk RM1, Snyder JA, Srinivasan P, et al. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J Pediatr Surg 2013;48:1:67-73.
11. Brullé L, Vandamme M, Riès D, et al. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS One 2012;7:12:e52653.
12. Partecke LI, Evert K, Haugk J, et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012;12:473.
13. Chang JW, Kang SU, Shin YS, et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch Biochem Biophys 2014;545:133-40.
14. Hirst AM, Frame FM, Maitland NJ, O’Connell D. Low temperature plasma: a novel focal therapy for localized prostate cancer? Biomed Res Int 2014;878319.