Clinical Findings
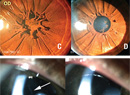
Manifestations of elevated IOP in children can vary depending on age of onset and rate of pressure elevation. Gradually increasing pressure can result in little to no corneal clouding. Presentation with buphthalmos and/or symptoms of tearing, blepharospasm and photophobia are more common (See Figure 1). In contrast, those children with acute pressure elevations present with corneal clouding. This finding can also be seen at birth (See Figure 2). Firm tactile pressure in these cases can be apparent and helpful in differentiating other causes of corneal opacification. The presence of a poor red reflex can elucidate subtle corneal clouding, although absence of a red reflex can be related to other pathology as well. Haab’s striae, which represent breaks in Descemet’s membrane, can be present in the absence of elevated pressure (See Figure 3). This finding signifies a history of elevated IOP associated with rapid eye growth.
|
An exam under anesthesia is essential in diagnosing childhood glaucoma. Pre-intubation IOP, refraction, axial length, corneal diameter, gonioscopy, and ultrasound biomicroscopy when visibility is poor, are key components of the exam. Progressive myopia, increasing axial length and changing corneal diameter in the face of borderline IOP and cupping are suggestive of fluctuating high pressures. Tracking these factors also aids in determining treatment response. In children more than 3 years of age, changes associated with buphthalmos become less apparent due to decreased scleral elasticity.6 With general anesthesia, a more thorough exam investigating risk factors for glaucoma such as signs of past trauma, uveitis and syndrome manifestations can be assessed.
A large number of syndromes have associated glaucoma. The more common syndromes are: Sturge-Weber; Oculocerebrorenal (Lowe); Axenfeld-Rieger; aniridia; and Neurofibromatosis Type 1.
Sturge-Weber has a sporadic inheritance pattern and is characterized by nevus flammeus (port wine stain) of the face, angioma of the meninges and, rarely, involvement of the airway. Incidence of glaucoma has been reported to be as high as 71 percent.7,8
|
Axenfeld-Rieger syndrome (ARS) is autosomal dominant but can occur sporadically.10 It is associated with anterior segment abnormalities and is often categorized under anterior segment dysgenesis or goniodysgenesis syndromes. Approximately 50 percent of patients diagnosed with ARS will develop glaucoma. Onset typically occurs during late childhood but can present during infancy and into adulthood.11 Physical manifestations include: redundant umbilical skin; telecanthus; broad nasal bridge; dental abnormalities (microdontia, oligodontia or hypodontia); and, in some cases, pituitary abnormalities with growth retardation.
Aniridia is characterized by hypoplastic iris tissue and is associated with foveal hypoplasia, cataracts, keratopathy secondary to limbal stem cell deficiency and, occasionally, optic nerve hypoplasia. The inheritance pattern is autosomal dominant, but can be inherited sporadically. In sporadic cases, patients should be worked up for Wilms’ tumor-aniridia-genital anomalies-retardation (WAGR) syndrome. Prevalance of glaucoma is reported to be from 30 to 50 percent.12
Neurofibromatosis Type 1 has an autosomal-dominant inheritance. It carries a spectrum of findings including café-au-lait spots, freckling of the axial/inguinal area, sphenoid dysplasia, S-shaped plexiform neurofibromas of the lids, optic nerve gliomas, Lisch nodules and choroidal hamartomas. Eyes with an associated plexiform neurofibroma have a 50 percent risk of glaucoma.13
Treatment
• Medical. Medical therapy in pediatric glaucoma is often supplementary to surgical management. It is often used for preoperative treatment to facilitate clearing of corneal edema. In addition, it can play a role in treating patients who are too unstable to undergo anesthesia. Timolol is often used as a first-line agent and has been shown to effectively lower IOP in the pediatric population. There is an increased risk for bronchospasm, apnea and bradycardia. The use of betaxolol (b1 selective antagonist), timolol 0.25% gel, and timolol 0.1% can help to avoid these side effects. Overall, however, timolol drops are generally well-tolerated.14 Latanoprost has been shown to have IOP-lowering effects, particularly in older children, but the non-response rate has been shown to be higher than in adults. Side effects are minimal, although darkening of the irides can occur, as in adults.15 Topical carbonic anhydrase inhibitors are also effective in lowering IOP. They are generally well-tolerated with minimal side effects. Oral acetazolamide has been shown to be more effective in lowering IOP and can be used in children with glaucoma at doses of 5 mg/kg/day to 15 mg/kg/day. Oral treatment carries a risk of systemic side effects, such as metabolic acidosis. Brimonidine has the most well-established side effect profile in children, causing bradycardia, hypotension, hypothermia, hypotonia and apnea in infants and severe lethargy in toddlers.16 Because of these side effects, its use is limited in the pediatric population.
• Surgical. Angle surgery is considered the mainstay of treatment for primary congenital glaucoma, with a reported 70 to 90 percent success rate after one to two procedures in patients treated after 3 months of age and before 1 to 2 years of age. This success rate significantly diminishes in patients presenting outside of this age range and those who fall in the spectrum of developmental glaucoma.17
|
Complications include misdirection into the suprachoroidal space, hyphema, cyclodialysis cleft and iris tear.19,20 The use of an illuminated microcatheter (iTRACK 250A; iScience Interventional) has been introduced to avoid misdirection. The illuminated catheter can be easily located. In addition, when 360 degree passage cannot be achieved, a second sclerotomy site can be created over the illuminated tip, allowing for partial treatment of the angle.21 Major complications for all angle surgeries include hyphema, hypotony and cataract.
Trabeculectomy has a 60 to 65 percent success rate when performed with antifibrotic agents. Success rates significantly decrease with aphakia. In addition, risk of bleb-related endophthalmitis in the pediatric population has been reported to be 7 to 14 percent.22 This risk appears to increase over time.23 Combination trabeculectomy-trabeculotomy has been described with a reported success rate of 72 percent as a primary procedure; however, it is unclear if there is a difference in success when comparing trabeculotomy alone vs. combined trabeculectomy/trabeculotomy.24
Aqueous shunt implantation has shown significantly greater success when compared to trabeculectomy.25 Low endophthalmitis rates have been reported. It does appear, as in adults, that implants become less effective over time and require reoperation.26 Implants available for use include: Ahmed valve (New World Medical); Baerveldt implant (Pharmacia); and Molteno implant (OP Inc). Ahmed valves and Baerveldt implants are the most commonly used and have both been reported to be effective.26-28
Cyclodestruction procedures are an option in difficult-to-treat cases. Cyclocryotherapy has been replaced by laser cyclophotocoagulation. A transscleral technique is most commonly used. Endoscopic cyclophotocoagulation has been reported to be effective as well.29
Prognosis
Reports of visual outcomes vary. Cases resulting in visual acuity sufficient to qualify for a motor vehicle driving license range from 29 to 46.6 percent of patients. Vision at the time of diagnosis, type of glaucoma and amblyopia appear to be the largest factors in visual outcomes. Children with primary congenital glaucoma have the best prognosis. In the setting of well-controlled intraocular pressure, amblyopia is a key factor in vision loss. As in pediatric patients with congenital cataracts, unilateral cases often have poorer visual outcomes secondary to amblyopia.30,31
Counseling patients and their families with regard to potential future vision loss can be challenging. Connecting them to resources for the visually impaired early is of utmost importance. The following are a few organizations with resources for the visually impaired and blind: Lighthouse International ( lighthouse.org); Helen Keller Services for the Blind ( helenkeller.org); American Foundation for the Blind ( afb.org); National Federation for the Blind ( nfb.org); and Family Connect ( familyconnect.org/parentsitehome.aspx). REVIEW
Dr. Huang is a pediatric ophthalmologist specializing in pediatric glaucoma, amblyopia, eye muscle disorders, pediatric cataracts, congenital blocked tear ducts and other pediatric eye conditions. She is a full-time faculty member of the New York Eye and Ear Infirmary of Mt. Sinai and an assistant professor of ophthalmology. She reports no financial interest in any of the products discussed.
1. Biglan AW. Glaucoma in children: Are we making progress? J AAPOS. 2006 Feb;10(1):7-21.
2. Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: A population-based study. Arch Ophthalmol 2010;128:478-82.
3. Bermejo E, Martinez-Frias ML. Congenital eye malformations: Clinical-epidemiological analysis of 1,124,654 consecutive births in Spain. Am J Med Genet 1998;75:497-504.
4. Lambert SR, Melia M, Buffenn AN, Chiang MF, et al. Rebound tonometry in children: A report by the American Academy of Ophthalmology. Ophthalmology 2013 Apr;120(4):e21-7. doi: 10.1016/j.ophtha.2012.09.058. Epub 2013 Feb 8.
5. Dahlmann-Noor AH, Puertas R, Tabasa-Lim S, El-Karmouty A, et al. Comparison of handheld rebound tonometry with Goldmann applanation tonometry in children with glaucoma: A cohort study. BMJ Open 2013 Apr 2;3(4).
6. Sampaolesi R. Congenital glaucoma. The importance of echometry in its diagnosis, treatment and functional outcome. In Cennamo G, Rosa N (eds): Ultrasonography in Ophthalmology London, England: Kluwer Academic Publishers, 1997:1-47.
7. Sharan S, Swamy B, Taranath DA, Jamieso R, et al. Port wine vascular malformation and glaucoma risk in Sturge-Weber Syndrome. J AAPOS 2009;13(4):374-8.
8. Sullivan TJ, Clarke MP, Morin JD. The ocular manifestations of the Sturge-Weber syndrome. J Pediatr Ophthalmol Strabismus 1992; 29(6):349-56.
9. Walton DS, Katsavounidou G, Lowe CU. Glaucoma with the oculocerebrorenal syndrome of Lowe. J Glaucoma 2005;14:181-5.
10. Alward WLM. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol 2000;130:107-115.
11. Chang TC, Summers CG, Schimmenti LA, Grajewski AL. Axenfeld-Rieger syndrome: New perspectives. Br J Ophthalmol 2012;96:318-22.
12. Hingorani M, Hanson I, van Heyningen V. Aniridia. Eur J Hum Genet 2012 Oct;20(10):1011-7. doi: 10.1038/ejhg.2012.100. Epub 2012 Jun 13. Review.
13. Sippel KC. Ocular findings in neurofibromatosis type 1. Int Ophthalmol Clin 2001;41(1):25-40. Review.
14. Plager DA, Whitson JT, Netland PA, Vijaya L, et al, BETOPTIC S Pediatric Study Group. Betaxolol hydrochloride ophthalmic suspension 0.25% and timolol gel-forming solution 0.25% and 0.5% in pediatric glaucoma: A randomized clinical trial. J AAPOS 2009;13(4):384-90.
15. Black AC, Jones S, Yanovitch TL, Enyedi LB, Stinnett SS, Freedman SF. Latanoprost in pediatric glaucoma--pediatric exposure over a decade. J AAPOS 2009;13(6):558-62.
16. Carlsen JO, Zabriskie NA, Kwon YH, et al. Apparent central nervous system depression in infants after the use of topical brimonidine. Am J Ophthalmol 1999;128:255-256.
17. Russell-Eggitt IM, Rice NSC, Jay B, et al. Relapse following goniotomy for congenital glaucoma due to trabecular dysgenesis. Eye 1992;6:197-200.
18. Joos KM, Alward WLM, Folberg R. Experimental endoscopic goniotomy: A potential treatment for primary infantile glaucoma. Ophthalmology 1993;100:1066-1070.
19. Neely DE. False passage: A complication of 360 degrees suture trabeculotomy. J AAPOS 2005;9:396-7.
20. Verner-Cole EA, Ortiz S, Bell NP, Feldman RM. Subretinal suture misdirection during 360 degrees suture trabeculotomy. Am J Ophthalmol 2006;141:391-2.
21. Girkin CA, Rhodes L, McGwin G, Marchase N, Cogen MS. Goniotomy versus circumferential trabeculotomy with an illuminated microcatheter in congenital glaucoma. J AAPOS 2012;16(5):424-7.
22. Beck AD. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am 2001;14(3):501-12.
23. Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmology 2000;107:422-9.
24. Mullaney PB, Selleck C, Al-Awad A, Al-Mesfer S, Zwaan J. Combined trabeculotomy and trabeculectomy as an initial procedure in uncomplicated congenital glaucoma. Arch Ophthalmol 1999;117:457-60.
25. Beck AD, Freedman S, Kammer J, Jin J. Aqueous shunt devices compared with trabeculectomy with mitomycin C for children in the first two years of life. Am J Ophthalmol 2003;136:994-1000.
26. O’Malley Schotthoefer E, Yanovitch TL, Freedman SF. Aqueous drainage device surgery in refractory pediatric glaucomas: I. Long-term outcomes. J AAPOS 2008 Feb;12(1):33-9. Epub 2007 Oct 17.
27. Morad Y, Donaldson CE, Kim YM, Abdolell M, Levin AV. The Ahmed drainage implant in the treatment of pediatric glaucoma. Am J Ophthalmol 2003;135:821-9.
28. Budenz D, Gedde S, Brandt J, Kira D, et al. Baerveldt glaucoma implant in the management of refractory childhood glaucomas. Ophthalmology 2004;111:2204-10.
29. Neely DE, Plager DA. Endocyclophotocoagulation for management of difficult pediatric glaucomas. J AAPOS 2001;5(4):221-9.
30. Khitri M, Mills M, Ying G, Davidson S, et al Visual acuity outcomes in pediatric glaucomas. J AAPOS 2012 Aug;16(4):376-81.
31. Kargi SH, Koc F, Biglan AW, Davis JS. Visual acuity in children with glaucoma. Ophthalmology 2006;113:229-38. Epub 2006 Jan 10.