There is also evidence that NSAIDS may be effective as primary or adjunctive therapy for the treatment of posterior segment disorders. The best known example is pseudophakic cystoid macular edema, for which NSAIDS have been shown to be effective both alone and as adjunctive therapy with steroids. CME is also associated with a variety of retinal disorders (See Table 2). NSAIDS have now been used "off-label" as adjunctive therapy for macular edema associated with, for example, diabetic retinopathy, retinal vein occlusions, uveitis, choroidal neovascularization and epiretinal membranes. Evidence for the efficacy of NSAIDS for the treatment of CME associated with these disorders, however, is limited.
This article will review evidence for the use of NSAIDS in the treatment of retinal disorders and some of the scientific rationale supporting their use.
Mechanism of Action
NSAIDs act by blocking the cyclooxygenase enzymes, COX-1 and COX-2. As shown in Figure 2, the COX-2 enzyme mediates the production of prostaglandins that contribute to the inflammatory response and ocular disease. The NSAIDs used to treat ophthalmic disorders inhibit both COX-1 and COX-2 enzymes with variable potency against each. As a rule, the inhibition of COX-2 determines the clinical efficacy of an ophthalmic NSAID.
Pseudophakic CME
Cataract surgery is associated with postoperative macular edema in up to 30 percent of cases. In most instances this edema is not clinically relevant and resolves spontaneously without supplemental therapy. In a small percentage of cases (1 to 2 percent), the edema becomes chronic and, untreated, may lead to permanent vision loss.
The mechanism of CME following cataract surgery is primarily related to the inflammation associated with the surgical procedure. The surgical incision, uveal stimulation and disruption of the lens capsule may all lead to release of prostaglandins and breakdown of the blood-aqueous barrier with release of cytokines and other inflammatory mediators. These mediators diffuse to the posterior pole where they increase vascular permeability in the retina causing a breakdown of the blood-retinal barrier (BRB) that leads to macular edema. Patients at increased risk for this break down of the BRB include elderly patients, diabetics and those with any of the conditions listed in Table 2.
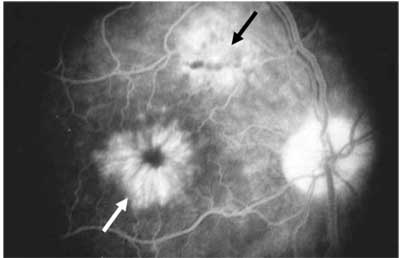
Figure 1. Late-phase fluorescein angiogram of pseudophakic CME (white arrow). Note the triangular filamentous window defect suggesting a microscope burn (black arrow).
The gold standard for diagnosing CME is the fluorescein angiogram. As shown in Figure 1, the late phase of the FA demonstrates cystic spaces in a flower-shaped ("petaloid") pattern. The FA provides direct evidence that CME, as a rule, is associated with an increase in vascular permeability. The angiogram gives both a qualitative and semi-quantitative assessment of CME. In addition to detecting CME, FA usually reveals the underlying cause—in this case, an apparent microscope burn.
Optical Coherence Tomography has become the most common way to screen for CME. OCT provides excellent quantitative data on the amount of CME but should not, as a rule, be used alone to diagnose the underlying cause of CME, since this may be missed on the OCT alone.
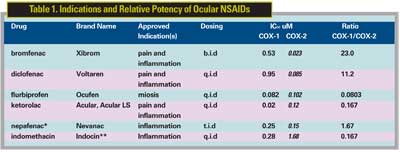
Topical steroids have been the standard of care for managing CME. Studies have shown, however, that NSAIDS are also effective in a synergistic way and the combination of NSAIDS and steroids may more effective than either drug alone. Limited studies for each of the major topical NSAIDS listed in Table 1 suggest some efficacy against pseudophakic CME, and evidence for superiority of one agent over another is not available. Studies suggest that prophylactic treatment with NSAIDS can reduce the incidence of postoperative CME. It is interesting to note that topical diclofenac and ketorolac have been found to be comparable to topical steroids in the treatment of pseudophakic CME in small uncontrolled studies. CME induced by topical prostanoids in pseudophakic patients also appears to be responsive to topical NSAID therapy. The lower the IC50, the higher the potency. The higher the ratio of COX1/COX2, the higher the theoretical effect on inflammation versus platelet inhibition and gastrointestinal prophylaxsis. Approved indications: pain following refractive surgery, inflammation following cataract and/or refractive surgery. (*Active form of nepafenac is amfenac and the IC50s shown are for amfenac. **Indocin is not commercially available in the United States but can be formulated as a 0.1% solution by a compounding pharmacy.)
Epiretinal Membranes
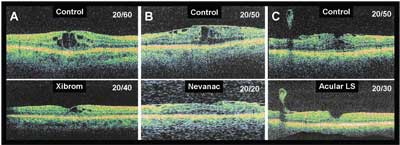
"Idiopathic" epiretinal membranes appear to result from glial cell proliferation on the retinal surface in response to defects in the internal limiting membrane created by a posterior vitreous detachment. Epiretinal membranes are often associated with macular thickening on OCT, although FA may or may not demonstrate active leakage. In cases in which active leakage on the FA is observed, the prognosis is usually worse, since persistent CME will eventually lead to degenerative changes in the retina and progressive vision loss. If vision loss is relatively mild (e.g., 20/25 to 20/50), I consider medical management with steroid or non-steroidal drops. Since long-term treatment may be required, I usually try an NSAID alone to avoid the risks of secondary glaucoma and cataract. In my experience, only a small percentage (<10 percent) of patients with ERM and CME on FA will respond to topical NSAIDS alone with a decrease in edema and improvement in vision. Figure 3 illustrates examples of macular edema reduced by three topical NSAIDs as measured by OCT. Note the accompanying improvement in visual acuity in each case. In these cases, the improvement in vision eliminated the need for surgical intervention. A controlled study is required to assess the efficacy, risks and benefits of NSAIDs in the treatment of macular edema associated with epiretinal membranes.
Retinal Vein Occlusions
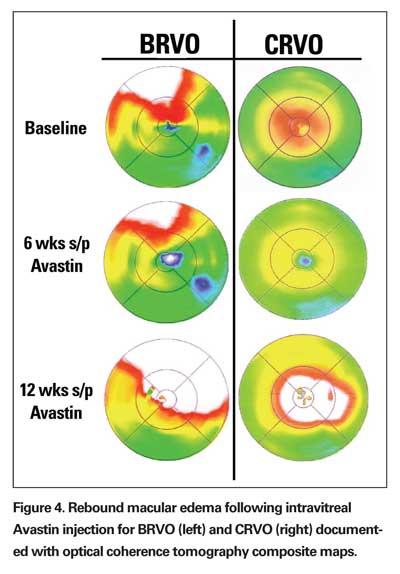
Branch and central retinal vein occlusions associated with vision loss are nearly always associated with CME. The CME is usually responsive to "off-label" intravitreal steroid (triamcinolone acetate) or the anti-vascular endothelial growth factor (VEGF) antibody, bevacizumab (Avastin, Genetech), but the effect is often transient and associated with rebound edema (See Figure 4). Follow-up treatment with grid or focal laser may reduce recurrence of edema, but recurrence is still common and this has not been formally studied.
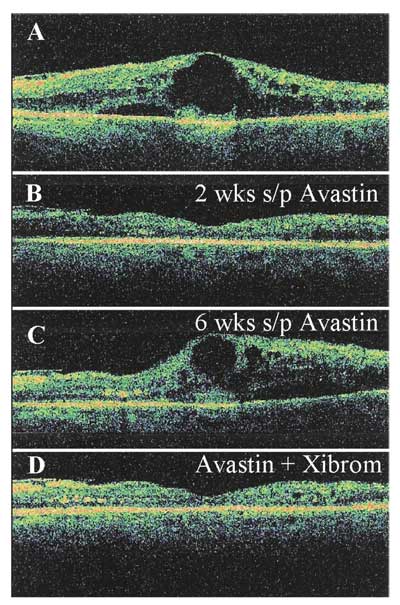
Topical NSAIDS may be used as adjunctive therapy, theoretically to reduce the chance of recurrent macular edema. An example of one case requiring multiple bevacizumab injections with subsequent stabilization on a NSAID drop alone is shown in Figure 5. Again, randomized clinical trial is needed to determine the efficacy, risks and benefits of this adjunctive therapy.
Diabetic Macular Edema
A subset of patients with clinically significant DME have associated CME. Many of these patients do not respond well to laser treatment alone. Adjunctive or primary therapy with intravitreal steroids or bevacizumab is often effective, though, as with vein occlusions, the reduction in CME and improvement in vision is often transient. Follow-up treatment with grid and focal laser may reduce the chance of recurrent leakage, but in many cases the edema still recurs. NSAIDS may reduce the incidence and severity of recurrent macular edema, and I often add an NSAID following an intravitreal injection or laser treatment for CSME associated with CME, particularly when the eye is pseudophakic. A randomized clinical trial is needed to determine the risks and benefits of topical NSAIDS for adjunctive therapy of CME in diabetic retinopathy.
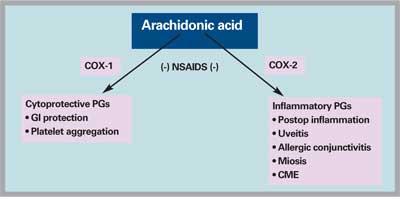
Figure 2. NSAIDs block the formation of certain prostaglandins through inhibition of the cyclooxygenase (COX) enzymes. COX-1 catalyzes the production of cytoprotective prostaglandins that coat the stomach lining with mucus (gastrointestinal or GI protection) and mediate platelet aggregation. COX-2 catalyzes the conversion of arachidonic acid into the inflammatory prostaglandins involved in postoperative inflammation, uveitis, allergic conjunctivitis, puppilary miosis and cystoid mascular edema (CME).
Macular Degeneration
A variety of conditions, including myopia, presumed ocular histoplasmosis syndrome (POHS) and aging, lead to macular degeneration, which remains the leading cause of blindness in the United States. Blindness in macular degeneration is most often the result of the growth of abnormal blood vessels beneath the retina called choroidal neovascular membrane. These membranes leak—hence the name "wet" macular degeneration. Vascular endothelial growth factor is a key player in the development of CNV associated with wet macular degeneration. As a result, anti-VEGF therapy is now the cornerstone of treatment for wet AMD and includes the off-label use of the full-sized anti-VEGF antibody, bevacizumab (Avastin, Genentech), the on-label use of the genetically engineered antibody fragment ranibizumab (Lucentis, Genentech) and the oligonucleotide "aptamer" pegaptanib (Macugen, OSI/Eyetech). Intravitreal steroid injections may also exert their effect in part by inhibition of VEGF.
Recent studies have shown that NSAIDS have anti-VEGF activity as well. This anti-VEGF activity may contribute to their efficacy in treating pseudophakic CME and suggests a possible adjunctive role in the management of patients with wet AMD.
Figure 3. Examples of macular edema associated with epiretinal membrane responding to topical treatment with NSAIDs. A) Xibrom applied b.i.d. for 10 weeks; B) Nevanac applied t.i.d. for 12 weeks; and C) Acular LS applied q.i.d. for 12 weeks. Note the accompanying improvements in visual acuities and the comparable improvements in macular edema. OCT images were from the same meridians for control and follow-up studies.
A clinical trial has already evaluated the efficacy of the NSAID diclofenac as an adjunctive therapy for patients with wet AMD undergoing photodynamic therapy with Visudyne. The limited study showed an initial trend for a beneficial effect but this was not sustained and did not reach statistical significance. More recent animal studies demonstrated inhibition by nepafenac of retinal angiogenesis, including CNV. Formal clinical studies will be required to assess the efficacy of topical NSAIDS as adjunctive therapy for retinal angiogenesis.
Currently, however, for pseudophakic patients with CNV associated with CME, I will add an NSAID as adjunctive therapy, since a component of pseudophakic CME cannot be ruled out.
Selecting an NSAID
A number of topical NSAIDS are now available for ophthalmic use and the most commonly used drugs, along with their on-label indications, are shown in Table 1. The most commonly prescribed NSAIDS and their relative market shares as of August 2006, based on Bank of America data are: Acular (23.9 percent; Acular LS 33.5 percent, both Allergan); Nevanac (21.3 percent, Alcon); and Xibrom (10.7 percent, Ista Pharmaceuticals). The major factors affecting selection include safety, efficacy, dosing frequency, tolerance, cost and availability.
• Safety. The safety of topical NSAIDS appears to be excellent, although there have been reports of significant side effects including corneal epitheliopathy, corneal melts, allergic conjunctivitis and systemic effects including GI upset and prolonged bleeding times. There is also evidence that NSAIDS could interfere with the intraocular pressure-lowering effects of prostanoids such as latanoprost.
Acular and Acular LS have the greatest number of prescriptions due in part to their long-term safety record (there are no documented cases of the above complications in clinical studies). Voltaren prescriptions fell when the generic product was introduced and with concomitant reports of corneal erosions and melts. Xibrom was also associated with a report of corneal melts—three cases reported in Japan in patients with pre-existing corneal disease. No case in the United States has been reported since the onset of its distribution here. Nepafenac is a pro-drug that is hydrolyzed into its active form (amfenac) within the eye and this may reduce surface toxicity related to the active drug. To reduce risk of ocular side effects from topical NSAIDS, avoid using in patients with severe dry eyes or a known allergy to the drug class or preservative. Follow the patients at weekly to monthly intervals depending on the duration of treatment and presence of any corneal changes.
• Efficacy. There are no large randomized, controlled clinical trials comparing the efficacy of topical NSAIDS for the treatment of ocular disorders. The relative potencies of the drugs, however, have been measured based on their abilities to inhibit the COX-1 and COX-2 enzymes, and are listed in Table 1. The inhibition of COX-2 is the basis for the anti-inflammatory effect of the drug and is thought to be primarily responsible for a drug's efficacy against ocular disorders.
Limited clinical data suggest comparable efficacy for the leading topical NSAIDS for postoperative pain, inflammation and CME. Pilot and retrospective studies have been reported for bromfenac, diclofenac, ketorolac and nepafenac. Inconsistent study designs make direct comparisons of NSAID efficacy for postop inflammation and pain difficult. Prospective, randomized, controlled studies are required to assess relative efficacy of NSAIDS for specific conditions.
• Dosing Frequency, Compliance. The newer NSAIDS, Xibrom and Nevanac, are dosed less frequently due in part to their greater apparent potency for inhibiting COX-2. Less frequent dosing, in turn, is known to improve compliance. Xibrom is dosed least frequently at b.i.d. and, when used in isolation, would provide the best compliance. Most NSAIDS, however, are used with an antibiotic during the postop period, and the t.i.d. dosing of Nevanac makes it a good choice with Vigamox, a popular fourth-generation fluoroquinolone used following intraocular and refractive surgical procedures. The majority of prescriptions for postop care are for topical antibiotics dosed q.i.d., however, which may account for the persistent popularity of Acular and Acular LS despite the q.i.d. dosing regimen.
• Tolerability. The main issues with tolerability have been burning and stinging with rates of up to 20 percent for Acular and Voltaren. The reformulation of Acular to Acular LS eliminated the stinging without affecting efficacy. The newer NSAIDS, Xibrom and Nevanac, are also well-tolerated.
• Cost and Availability. These factors still play a large role in drug selection. Acular and Acular LS continue to have the lowest cost and greatest availability with the broadest coverage on insurance plans.
Topical Versus Systemic Therapy
A few reports suggest that systemic COX-2 inhibitors may reduce the risk of postoperative CME following cataract surgery. Another report, however, did not confirm this benefit. Given the potential side effects associated with the use of systemic NSAIDS and the apparent weaker effect of systemic NSAIDS compared to topical drops for treating intraocular inflammation, systemic NSAIDS are unlikely to play a role in the management of most patients. In the case of patients with vein occlusions, selective COX-2 inhibitors should be avoided due to the theoretical increased risk of thrombosis. Nonselective NSAIDS, such as indomethacin, may be beneficial in the treatment of retinal disorders associated with systemic disease, such as posterior scleritis.
Figure 5. Response of macular edema from BRVO to Avastin injections as measured with OCT. A) Baseline OCT measurements. B) Decrease in macular edema two weeks following initial Avastin injection. C) Recurrence of macular edema six weeks following fist Avastin injection. D) Decrease in macular edema 12 weeks following second Avastin injection and initiation of topical treatment with bromfenac (Xibrom) b.i.d. immediately following second injection.
NSAIDS will play an increasing role in the management of both anterior segment and posterior segment disorders. In addition to prophylaxis against and treatment of pseudophakic CME, NSAIDS may also be used as adjunctive therapy for macular edema associated with other disorders, including diabetic retinopathy, retinal vein occlusions and macular degeneration. Recent studies suggest that the efficacy of topical NSAIDS for retinal disorders will relate to their ocular penetration, inhibition of the inflammatory mediators, and inhibition of VEGF. Randomized controlled clinical trials will be required to assess the relative efficacy, risks and benefits of NSAIDS as adjunctive therapy for retinal disorders. Improved delivery mechanisms, such as transcleral iontophoresis, depot injections and implantable devices, may increase the efficacy of this drug class for treating retinal disorders. In the meantime, rational treatment protocols for macular edema may include the judicious use of topical NSAIDS such as Acular, Acular LS, Nevanac and Xibrom.
Dr. Gallemore is an assistant clinical professor of ophthalmology at the Jules Stein Eye Institute, UCLA School of Medicine, and practices at Retina Vitreous Associates Medical Group. Contact him at (213) 483-8810 or Retina2000@yahoo.com.
Riendeau D, Charleson S, Cromlish W, Mancini JA, et al. Comparison of the cyclooxygenase-1 inhibitory properties of nonsteroidal anti-inflammatory drugs (NSAIDs) and selective COX-2 inhibitors, using sensitive microsomal and platelet assays. Can J Physiol Pharmacol 1997;75:1088-95.
Singal N, Hopkins J. Pseudophakic cystoid macular edema: ketorolac alone vs. ketorolac plus prednisolone. Can J Ophthalmol 2004;39:245-50.
Heier JS, Topping TM, Baumann W, Dirks MS, Chern S. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology 2000;107:2034-8;discussion 2039.
Meyer CH, Schmidt JC, Rodrigues EB, Mennel S. Risk of retinal vein occlusions in patients treated with rofecoxib (Vioxx). Ophthalmologica 2005;219: 243-7.
Altintas O, Yuksel N, Karabas VL, Demirci G. Cystoid macular edema associated with latanoprost after uncomplicated cataract surgery. Eur J Ophthalmol 2005;15:158-61.
Rho DS. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg 2003;29:2378-84.
Ursell PG, Spalton DJ, Whitcup SM, Nussenblatt RB. Cystoid macular edema after phacoemulsification: Relationship to blood-aqueous barrier damage and visual acuity. J Cataract Refract Surg 1999;25:1492-7.
van Haeringen NJ, van Sorge AA, Carballosa Core-Bodelier VM. Constitutive cyclooxygenase-1 and induced cyclooxygenase-2 in isolated human iris inhibited by S(+) flurbiprofen. J Ocul Pharmacol Ther 2000;16:353-61.
Gamache DA, Graff G, Brady MT et al. Nepafenac, a unique nonsteroidal prodrug with potential utility in the treatment of trauma-induced ocular inflammation: I. Assessment of anti-inflammatory efficacy. Inflammation 2000;24:357-70.
Guex-Crosier Y. Non-steroidal anti-inflammatory drugs and ocular inflammation. Klin Monatsbl Augenheilkd 2001;218:305-8.
Waterbury LD, Silliman D, Jolas T. Comparison of cyclooxygenase inhibitory activity and ocular anti-inflammatory effects of ketorolac tromethamine and bromfenac sodium. Curr Med Res Opin 2006; 22:1133-40.
Yang R, et al. Inhibition of VEGF–Induced Endothelial Cell Proliferation and Differentiation by Steroidal and Non–steroidal Cox Inhibitors with Variable Cox–1/Cox–2 Selectivity. IOVS 2004;45: E-Abstract 1914.
Rho DS, et al. Bromfenac 0.09% versus Diclofenac Sodium 0.1% versus Ketorolac Tromethamine 0.5% in the Treatment of Acute Pseudophakic Cystoid Macular Edema. IOVS 2006;47: E-Abstract 5211.
Wilkinson-Berka JL, Alousis NS, Kelly DJ, Gilbert RE. COX-2 inhibition and retinal angiogenesis in a mouse model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 2003;44:974-9.
Takahashi K, Saishin Y, Mori K, Ando A, et al. Topical nepafenac inhibits ocular neovascularization. Saishin Y. Invest Ophthalmol Vis Sci 2003;44:409-15.
Lukiw WJ, Ottlecz A, Lambrou G, Grueninger M, et al. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest Ophthalmol Vis Sci 2003;44:4163-70.
Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use. Experience and incidence in a retrospective review of 94 patients. Ophthalmology 1998;105:263-8.
Wright PL, Wilkinson CP, Balyeat HD, Popham J, Reinke M. Arch. Angiographic cystoid macular edema after posterior chamber lens implantation. Arch Ophthalmol 1988;106:740-4.
Chu YR, et al. Clinical Evaluation of Ocular Surface Toxicity of Ketorolac Tromethamine 0.4% (Acular LS) vs. Bromfenac 0.09% (Xibrom) Ophthalmic Solution. IOVS 2006;47: E-Abstract 74.
Ogawa T, et al. Pharmacokinetic Profile of Topically Applied Bromfenac Sodium Ophthalmic Solution 0.1% in Subjects Undergoing Cataract Surgery. IOVS 2006 47: E-Abstract 687.
Donnenfeld ED, Perry HD, Wittpenn JR, Solomon R, et al. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: Pharmacokinetic-response curve. J Cataract Refract Surg 2006;32:1474-82.
Solomon KD, Donnenfeld ED, Raizman M, et al. Safety and efficacy of ketorolac tromethamine 0.4% ophthalmic solution in post-photorefractive keratectomy patients. J Cataract Refract Surg 2004;30:1653-60.
Asai T, Nakagami T, Mochizuki M, Hata N, et al.Three cases of corneal melting after instillation of a new nonsteroidal anti-inflammatory drug. Cornea 2006;25:224-7.
Kashiwagi K, Tsukahara S. Effect of non-steroidal anti-inflammatory ophthalmic solution on intraocular pressure reduction by latanoprost. Br J Ophthalmol 2003;87:297-301.
Biswas PS, Banerjee K, Kim B, Kinchington PR, Rouse BT.Role of inflammatory cytokine-induced cyclooxygenase 2 in the ocular immunopathologic disease herpetic stromal keratitis. J Virol. 2005 Aug;79(16):10589-600. Erratum in: J Virol 2005;79:15590.




