Tear Markers and Keratoconus
Rohit Shetty, DNB, FRCS, PhD, is a cornea and refractive surgeon and a clinical and translational scientist working at Narayana Nethralaya Rajajinagar in Bangalore, India. Although keratoconus is not generally thought of as an inflammatory disease, Dr. Shetty’s work has supported the connection between inflammatory cytokines in the tear film and progressive keratoconus. His team has shown that keratoconus patients’ tears contain high levels of matrix metalloproteinase 9 (MMP9) and IL6 (one of the interleukin family of cytokines), both associated with inflammation, and that these levels correlate with disease progression. In addition, one recent study conducted by Dr. Shetty and colleagues demonstrated that the elevated levels of MMP9 and inflammatory cytokines in the tears of keratoconus patients could be reduced with cyclosporine treatment, leading to a concomitant arrest of disease progression.1
“My interest in this idea was the result of a few papers I’d seen on the connection between inflammation and keratoconus,” he explains. “Eye-rubbing, allergy and dry eye have all tentatively been linked to keratoconus, and these are often linked to inflammation. So we started our research with the question ‘Is keratoconus inflammatory?’ We began searching for tear biomarkers; this took time, but eventually we were able to prove that these biomarkers do exist.” Dr. Shetty says that their work has also demonstrated that progressive keratoconus has a unique inflammatory signature which differs from that of nonprogressive keratoconus. “The markers associated with progressive keratoconus cause more damage to the collagen and lysyl oxidase, which is necessary for cross-linking, both natural and artificial,” he says.
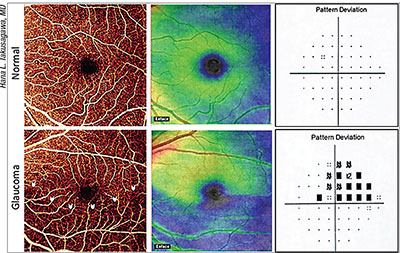 |
| Perfusion alterations in the superficial vascular complex of the macula—detectable using OCT angiography—show high sensitivity for differentiating glaucomatous eyes from normals, and correlate well with visual fields. Above: En face OCT angiograms, ganglion cell complex thickness maps and visual fields for a normal and a glaucomatous eye. In the glaucomatous eye, the angiogram and thickness map reveal an inferior arcuate defect (arrows); the visual field shows a superior defect at the same location. |
“The main message from our work is that keratoconus is an inflammatory disease,” says Dr. Shetty. “Surgeons should think of keratoconus as ‘white inflammation’—i.e., inflammation without cardinal signs such as redness and pain. Despite the lack of these signs, it’s still creating changes in the nerves and collagen. For that reason, it makes sense to treat any inflammation that’s present before doing any surgery or cross-linking. This is especially important in children. It’s also worth testing the levels of MMP9 before you perform cross-linking, to ensure that levels are normal. Then, after surgery or cross-linking, it’s important to continue to treat any inflammation with a drug such as cyclosporine for at least six months.
“It’s especially important for American surgeons to understand this, now that cross-linking has been approved in the United States,” he adds.
Dark Adaptation and AMD
It’s been known for some time that abnormal dark adaptation is an early sign of retinal trouble. The problem has been testing it; in the past, a patient had to sit in the dark for a half hour or more prior to lengthy testing, making it clinically impractical. Now a device called the AdaptDx (MacuLogix, Middletown, Pa.) can reliably test a patient’s dark adaptation function in six and a half minutes or less, making it a practical in-office test with multiple uses—in particular, detecting early evidence of age-related macular degeneration. (The test is Medicare reimbursable.)
Gregory R. Jackson, PhD, chief scientific officer at MacuLogix, says the device is easy to use and operator- and patient-friendly. “The device is also easy to interpret,” he notes. “The machine gives the doctor a printout showing a parameter we call the rod intercept, which is the amount of time it takes for you to have nearly complete recovery of rod function. If the rod intercept is less than six and a half minutes, it’s likely your macula is normal; if it’s over six and a half minutes, it’s likely your macula is abnormal.”
Dr. Jackson says that a number of studies have demonstrated that dark adaptation is highly sensitive and specific for age-related macular degeneration.2,3 “In a multisite clinical evaluation study, we found that dark adaptation was 90 percent accurate in classifying patients as having AMD or normal retinal health,” he says.2 “When patients were followed over a period of years, those with impaired dark adaptation in early testing were very likely to develop age-related macular degeneration.
“In 2016, Cynthia Owsley, PhD, at the University of Alabama Birmingham published the ALSTAR study,” he continues. “In this study, they enrolled 325 normal adults age 60 or older whose retinal health was verified by AREDs grading of fundus photographs. At baseline, 24 percent of those patients had clinically impaired dark adaptation, the first symptom of age-related macular degeneration. Three years later, they redid the imaging and graded the fundus photographs in a masked fashion using the AREDs criteria, and found that if you had impaired dark adaptation at baseline, you were about twice as likely to have clinically evident macular degeneration at three years of follow-up. Furthermore, patients with impaired dark adaptation at baseline were eight times as likely to progress from normal retinal health to intermediate macular degeneration.”4 (Dr. Owsley is the patent holder for the AdaptDx technology.)
Dr. Jackson says the reason for this is now well understood. “Work by Christine Curcio, PhD, at the University of Alabama Birmingham—among others—has shown that before you have visible drusen in a macular degeneration patient, deposits of cholesterol coat the back of the macula, impeding the transport of nutrients into the eye, including vitamin A.5 The resulting localized deficiency of vitamin A in their eyes slows the regeneration of rhodopsin, and thus dark adaptation. By the time you see one large druse in the back of the eye of an early macular degeneration patient, the whole back of the macula is coated with these cholesterol deposits, gumming up the works, denying the retina nutrition and keeping oxygen from the choroidal vasculature, impairing dark adaptation.”
Dr. Jackson says that doctors using the AdaptDx are excited to have functional information about these patients. “Not only does the AdaptDx help doctors identify patients with early disease, it also allows them to baseline their existing macular degeneration patients so they can tell whether the patient is doing better or worse,” he says. “We know from the Lucentis clinical crossover studies that if you’re seeing a patient every six months and the patient develops choroidal neovascularization shortly after a visit, the patient can lose five lines of visual acuity before the next visit. If you see impaired dark adaptation, you can be more proactive and tighten up the observation period.”
Dr. Jackson says he wants to be clear that dark adaptation can’t predict the onset—or severity—of choroidal neovascularization or geographic atrophy. “It gives you a more complete picture of the retinal health of the patient,” he says. “If you have a number of patients with intermediate macular degeneration, some will have much worse dark adaptation than others. Up until today, we’ve been using visual acuity as the canary in the coal mine; we don’t get concerned until visual acuity drops substantially. As a result, we do a really bad job of saving visual acuity in the first eye. Having this new information lets us use night vision as the canary in the coal mine.”
Dr. Jackson points out another recurring problem: patients referred to a cataract surgeon because of diminished vision that was actually caused by macular degeneration. “Many of our doctors are using the AdaptDx to help make a differential diagnosis,” he says. “Dark adaptation as measured by the AdaptDx is not affected by cataract, so if you test a patient’s dark adaptation and it’s normal, you’re probably looking at an optical cause for their acuity detriment or night vision problem. However, if the dark adaptation is impaired, you have to consider that you may be looking at a retinal problem.”
Dr. Jackson says the AdaptDx might also help a surgeon decide if someone is a good candidate for a multifocal IOL. “Multifocal IOLs reduce the amount of light reaching the retina,” he notes. “So if a patient has impaired night vision, many doctors think it’s not a good idea to put this type of lens into that eye.”
Dr. Jackson says the AdaptDx costs $39,900, and is most useful for a primary care ophthalmologist. “Ophthalmologists can use dark adaptation to test their older and at-risk patients to find out whether they have subclinical macular degeneration or macular disease that they might otherwise have missed,” he says.
Glaucoma and Macular Perfusion
Hana L. Takusagawa, MD, a glaucoma specialist and assistant professor at Oregon Health & Science University, has been working with David Huang, MD, PhD, and his team at the Center for Ophthalmic Optics and Lasers at the Casey Eye Institute, evaluating the potential for using OCT angiography to detect early glaucomatous damage in the upper layers of the macula.
“Glaucoma specialists who are interested in perfusion tend to study the optic nerve because that’s where obvious clinical signs of glaucoma such as cupping happen,” she says. “This new technology, OCT angiography, allows us to look at blood vessels in a quick, noninvasive way, without having to inject any dyes into the blood. Using the AngioVue OCT angiography system [Optovue, Fremont, Calif.], we’ve been comparing the perfusion in patients with glaucoma and those with healthy eyes. [Note: OCT angiography can also be done using the Zeiss Angioplex (Carl Zeiss Meditec, Dublin, Calif.)] Early on, we found that there’s significantly decreased perfusion in the optic nerve. Then, in a later paper we looked at the area around the optic nerve, the peripapillary retina, which shows decreased perfusion in glaucoma as well.
“Most recently, we’ve looked at the macula,” she continues. “We found that perfusion at the macula is also significantly decreased in people with glaucoma. About 30 percent of the retinal ganglion cells live in the macula, and those are the cells that are damaged in glaucoma; that may turn out to be the area in which we’ll see the earliest changes from glaucoma.”
Dr. Takusagawa explains that their most recent work has found that the superficial layer of the macula—the superficial vascular complex—shows much more blood vessel alteration in glaucoma patients than the deeper levels of the macula. “We’ve shown that there are four major plexuses of blood vessels in the retina,” she notes. “It’s the uppermost blood vessel complexes that are affected in glaucoma.”
One advance that has helped them detect this difference is an algorithm created by Dr. Huang’s team that removes flow projection artifacts from the scans. “OCT angiography detects motion based on changes in the OCT signal over time,” Dr. Takusagawa explains. “It detects flow signals in blood vessels, but also shows flickering shadows projected on deeper structures. This makes it hard to get clean measurements of blood flow in different levels of the retina. So, we’ve done our studies using an algorithm that removes those projections. At the moment, the algorithm is not part of any commercial device, but I’m sure it will become available at some point in the future.
“At the same time, we also looked at the all-layer macular blood flow, which the OCTA instruments can determine without using these algorithms,” she continues. “We found that all-layer retinal blood flow was decreased in the macula in glaucomatous eyes as well, so that measurement might also be clinically useful. However, just looking at the superficial layer of the macula was better at differentiating normal from glaucomatous eyes.” (In their study of 30 glaucoma patients and 30 age-matched normal participants, reported at the 2016 annual meeting of the American Glaucoma Society, when specificity was fixed at 95 percent, the sensitivity of superficial vascular complex vessel density for differentiating glaucomatous eyes from normal eyes was 90 percent; in contrast, the sensitivity was 80 percent using all-layer retinal vessel density, and 77 percent when using GCC thickness.)
“The other exciting thing about OCT angiography is that our findings correlate really well with the patient’s visual fields,” she says. “I can envision that OCTA might someday become a quantitative stand-in for the visual field test, which most patients dislike. Also, a lot of damage happens before a deficit is detectable on visual field testing; it’s possible that quantifying the vessel density in the upper layers of the macula might allow us to pick up damage earlier. Of course, it could turn out that OCT angiography measurements of the upper macula layers will be most effective when combined with other OCT measures like structural information about the ganglion cell complex.”
In terms of the future, Dr. Takusagawa says the team has been conducting a longitudinal trial using OCT angiography on both glaucoma suspects and those with confirmed glaucoma to determine the effectiveness of this technology for following progression. “Hopefully we’ll be coming out with some data about that in the next year or two,” she says. REVIEW
Dr. Shetty has research grants from Allergan and Zeiss.
1. Shetty R, Ghosh A, Lim RR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci 2015;56:2:738-50.
2. Jackson GR, Scott IU, et al. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmologist Vis Sci 2014;55:3:1427-31.
3. Owsley C, Huisingh C, Clark ME, et al. Comparison of visual function in older eyes in the earliest stages of age-related macular degeneration to those in normal macular health. Curr Eye Res 2016;41:2:266-72.
4. Owsley C, McGwin G, Clark ME, et al. Delayed rod-mediated dark adaptation Is a functional biomarker for incident early age-related macular degeneration. Ophthalmology 2016;123:2:344–351.
5. Jackson GR, Owsley C, Curcio CA. Photoreceptor degeneration and dysfunction in aging and age-related maculopathy. Ageing Res Rev 2002;1:381-386.



