Workup, Diagnosis & Discussion
Fundus photography was performed (Figure 1). Optical coherence tomography showed multiple areas of fresh subretinal fluid and macular edema (Figure 2). Fluorescein angiography in the right eye revealed extensive multifocal sites of early hyperfluorescence, presumed to represent “window defects” of retinal
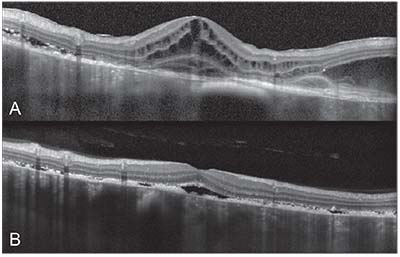 |
| Figure 2. Spectral domain optical coherence tomography of the right and left eyes. A) The right eye demonstrates multiple areas of subretinal and intraretinal fluid with focal destruction of the retinal layers and nodularity of the retinal pigment epithelium. B) The left eye demonstrates multiple areas of subretinal fluid with RPE nodularity. Note the diffuse choroidal thickening with loss of choroidal vascular details, suggestive of infiltration. |
pigment epithelial loss. There was circumpapillary staining. The pigmented choroidal lesions were hypofluorescent (Figure 3). Similar findings were noted in the left eye.
In summary, this patient had stage IV esophageal cancer and new-onset bilateral multifocal pigmented choroidal lesions with subretinal fluid, cystoid macular edema, extensive RPE mottling and prominent subretinal orange lipofuscin pigment. The differential diagnosis included:
• multifocal choroidal nevi;
• melanoma;
• metastases;
• bilateral diffuse uveal melanocytic proliferation (BDUMP);
• cancer-associated retinopathy;
• acute exudative polymorphous paraneoplastic vitelliform maculopathy; and
• medication-associated retinopathy from docetaxel.
Given the bilateral multifocal pigmented choroidal tumors and associated rapid-onset cataracts and conjunctival injection, the leading diagnosis was BDUMP.
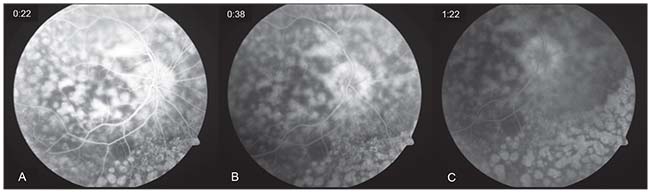 |
| Figure 3. Fluorescein angiography of the right eye. Early-phase angiogram (A) revealing a “window defect” hyperfluorescence of the multiple nummular retinal pigment epithelial atrophic lesions. Mid- (B) and late-phase (C) angiograms showing persistent hyperfluorescence of RPE defects and mild staining. Similar findings were observed in the left eye. |
Plasmapheresis was considered, but after discussion with the patient’s primary oncologist, the patient’s clinical status was considered inappropriate for plasmapheresis at that time. The patient declined to undergo anti-retinal antibody testing. He was started on oral prednisone 80 mg per day. Follow-up one month later revealed persistent poor vision of counting fingers in the right eye, with a decline to 20/150 in the left eye. The fundoscopic examination revealed unchanged findings. The patient was tapered off the oral corticosteroids and started on an intraocular corticosteroid with 2 mg of intravitreal triamcinolone. The patient was subsequently lost to follow-up.
Discussion
BDUMP is a rare paraneoplastic syndrome resulting in severe bilateral vision loss and proliferation of choroidal melanocytes. Retina specialist J. Donald Gass and co-workers described the five cardinal signs for the diagnosis of BDUMP, including: 1) multiple, subtle, round, orange-red subretinal patches in the fundus; 2) multifocal early hyperfluorescence of these patches on fluorescein angiography; 3) focally elevated pigmented and non-pigmented uveal melanocytic tumors with diffuse choroidal thickening; 4) exudative retinal detachment; and 5) rapidly progressive cataract formation.1 A characteristic ‘‘giraffe pattern’’ on fundus autofluorescence can be seen in BDUMP. It is believed to be secondary to nummular or polygonal RPE alterations and lipofuscin accumulation.2,3
In nearly half of the cases of BDUMP, there is a current or remote diagnosis of non-ocular malignancy at the time of diagnosis.4 BDUMP has no gender predilection and is associated with multiple visceral malignancies including cancer of the lung, colon, pancreas, gallbladder, ovary, uterus and cervix.4 Salient aspects of its causes and management include the following:
• Pathogenesis. The pathogenesis of BDUMP is poorly understood, but multiple theories have been suggested.5-7 One proposed hypothesis is that there may be production of melanocytic growth factors by the remote cancer cells with subsequent release into the circulation. A group of investigators recently studied the serum of patients with BDUMP and isolated an IgG antibody called cultured melanocyte elongation and proliferation (CMEP) factor that was involved in human melanocyte proliferation.5 This proliferation was found to be selective for melanocyte growth factor, since other cell lines—including human dermal fibroblasts, keratinocytes and ovarian cancer cells—that were treated with plasma from BDUMP patients containing the CMEP factor didn’t show proliferation.
Another study further supported these findings by demonstrating changes in melanocyte proliferation based on the presence or absence of the CMEP factor in BDUMP patients undergoing plasmapheresis.8 In this study, the plasma of a patient with BDUMP before systemic treatment induced growth of cultured melanocytes, confirming the presence of the CMEP factor; however, plasma from a patient treated with plasmapheresis did not induce melanocyte proliferation.8 Collectively, these findings suggested that treatment of the underlying malignancy and plasmapheresis eliminated CMEP and potentially reduced ocular manifestations of BDUMP. Indeed, the presence of the circulating CMEP factor may explain why approximately 26 percent of BDUMP patients have pigmented lesions in extraocular tissues such as the skin and mucous membranes.5 Our patient declined to undergo serum testing due to cost considerations.
Other studies have suggested a possible role of anti-retinal antibodies in photoreceptor destruction, as these circulating antibodies have been detected in BDUMP patients; however, their significance is unclear as these studies were confounded by the presence of multiple paraneoplastic retinopathies.7,9 Some investigators have proposed that there may be a common oncogenic stimulus initiating both the ocular and nonocular tumors.6,7
• Management. Treatment options for BDUMP are poorly understood and produce variable responses. As BDUMP is stimulated by a systemic malignancy, varied treatments of the primary malignancy by combinations of surgery, radiation and/or chemotherapy may confound reported visual outcomes. Based on a recent review article, the treatment options for BDUMP include no treatment, primary tumor treatment, intraocular surgery (e.g., retinal detachment surgery with subretinal fluid drainage), ocular radiation, local and systemic steroid treatment, cataract surgery, intravitreal bevacizumab injection and plasmapheresis.10
BDUMP is partly characterized by cataract formation, which can occur rapidly. This is hypothesized to result from ciliary body tumor invasion, leading to inadequate aqueous volume or poor nutrient composition.11 Cataract surgery is a common treatment option and serves as a temporizing measure for visual acuity improvements but hasn’t been shown to improve vision permanently when used as a sole intervention.4,10,11
Recently, plasmapheresis has emerged as a promising novel treatment for improving visual acuity in patients with BDUMP. Based on the suspicion of a circulating CMEP factor, plasmapheresis theoretically could remove the inciting factor from the serum. There have been several cases identified in the literature employing plasmapheresis for the treatment of BDUMP.8,9,12-18 One group postulated the use of plasmapheresis in BDUMP and described an example of a 71 year-old woman with metastatic papillary serous adenocarcinoma of the endometrium with a visual acuity of 20/40 OU and fundoscopic findings consistent with BDUMP.13 She underwent a course of plasmapheresis with seven volume exchange sessions every other day. She continued on the chemotherapy regimen for malignancy control and eventually underwent bilateral cataract extraction. At 13-month follow up, the pigmented fundus lesions remained stable in size, and her visual acuity was 20/25 OU. Another study documented a similar case of a 59 year-old woman with metastatic ovarian cancer and visual acuity of 20/150 OU who was diagnosed with BDUMP.14 The patient declined chemotherapy and underwent plasmapheresis three times per week and reported subjective improvement in her vision in both eyes at four months follow-up.
In summary, BDUMP should be considered in the differential diagnosis of patients with bilateral multifocal pigmented choroidal lesions, whether or not a known history of malignancy exists. Patients with a diagnosis of BDUMP have a poor prognosis, but novel treatments continue to evolve in order to improve their quality of life and visual outcomes. REVIEW
1. Gass JD, Gieser RG, Wilkinson CP, Beahm DE, Pautler SE. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol 1990;108:4:527-533.
2. Wu S, Slakter JS, Shields JA, Spaide RF. Cancer-associated nummular loss of the pigment epithelium. Am J Ophthalmol 2005;139:5:933-935.
3. Rahimy E, Soheilian M. Giraffe pattern of bilateral diffuse uveal melanocytic proliferation. Ophthalmology 2016;123:3:483.
4. Rahimy E, Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: Evaluation and management. Surv Ophthalmol 2013;58:5:430-458.
5. Miles SL, Niles RM, Pittock S, et al. A factor found in the igG fraction of serum of patients with paraneoplastic bilateral diffuse uveal melanocytic proliferation causes proliferation of cultured human melanocytes. Retina 2012;32:9:1959-1966.
6. Barr CC, Zimmerman LE, Curtin VT, Font RL. Bilateral diffuse melanocytic uveal tumors associated with systemic malignant neoplasms. A recently recognized syndrome. Arch Ophthalmol 1982;100:2:249-255.
7. Saito W, Kase S, Yoshida K, et al. Bilateral diffuse uveal melanocytic proliferation in a patient with cancer-associated retinopathy. Am J Ophthalmol 2005;140:5:942-945.
8. Jansen JC, Van Calster J, Pulido JS, et al. Early diagnosis and successful treatment of paraneoplastic melanocytic proliferation. Br J Ophthalmol 2015;99:7:943-948.
9. Mets RB, Golchet P, Adamus G, et al. Bilateral diffuse uveal melanocytic proliferation with a positive ophthalmoscopic and visual response to plasmapheresis. Arch Ophthalmol 2011;129:9:1235-1238.
10. Moreno TA, Patel SN. Comprehensive review of treatments for bilateral diffuse uveal melanocytic proliferation: A focus on plasmapheresis. Int Ophthalmol Clin 2017;57:1:177-194.
11. O’Neal KD, Butnor KJ, Perkinson KR, Proia AD. Bilateral diffuse uveal melanocytic proliferation associated with pancreatic carcinoma: A case report and literature review of this paraneoplastic syndrome. Surv Ophthalmol 2003;48:6:613-625.
12. Navajas EV, Simpson ER, Krema H, et al. Cancer-associated nummular loss of RPE: Expanding the clinical spectrum of bilateral diffuse uveal melanocytic proliferation. Ophthalmic Surg Lasers Imaging 2011;42:e103-106.
13. Jaben EA, Pulido JS, Pittock S, Markovic S, Winters JL. The potential role of plasma exchange as a treatment for bilateral diffuse uveal melanocytic proliferation: A report of two cases. J Clin Apher 2011;26:6:356-361.
14. Alasil T, Coady PA, Koudsi S, Mathur M, Materin MA. Bilateral diffuse uveal melanocytic proliferation: A case report. Retin Cases Brief Rep 2017;11:1:71-74.
15. Schelvergem KV, Wirix M, Nijs I, Leys A. Bilateral diffuse uveal melanocytic proliferation with good clinical response to plasmapheresis and treatment of the primary tumor. Retin Cases Brief Rep 2015;9:2:106-108.
16. Alrashidi S, Aziz AA, Krema H. Bilateral diffuse uveal melanocytic proliferation: A management dilemma. BMJ Case Rep. May 22, 2014. doi: 10.1136/bcr-2014-204387.
17. Pulido JS, Flotte TJ, Raja H, et al. Dermal and conjunctival melanocytic proliferations in diffuse uveal melanocytic proliferation. Eye (Lond) 2013;27:9:1058-1062.
18. Katz MSJ, Leder HA, Choudhury T. Successful treatment of bilateral diffuse uveal melanocytic proliferation with plasmapheresis. Journal of VitreoRetinal Diseases 2017:2474126417724656.



