LASIK
Minimal pain and quick recovery have made LASIK the most popular laser refractive surgery in the United States. Taking the time to go through key aspects of the operation will ensure it’s a successful procedure with your patients, as well.
• Preop exam. A thorough physical exam of the eye, including a dilated exam, imaging, pachymetry, topography and wavefront aberrometry will help you determine if the patient
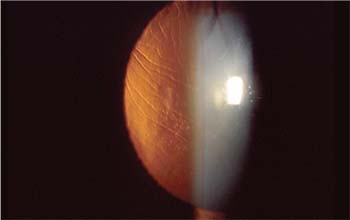 |
| Flap wrinkles are most effectively treated when addressed early in the postop course. |
• Motivations and expectations. Though the results of the physical exam can put a hard stop to any refractive surgery, your discussion with the patient regarding his motivations for the surgery is equally important. Someone can be a good candidate on paper but then, when you have a conversation and find out what he’s looking for, you might discover he doesn’t have realistic expectations.
A common example is the 45-year-old who wears -1 D glasses but doesn’t wear reading glasses and has never worn contact lenses. She wants crystal-clear distance vision and doesn’t understand the concept of presbyopia. In cases like this, I’ll demonstrate presbyopia in the office, or fit her with contact lenses to demonstrate it. When I do this, in many cases these patients decide not to have the surgery, since it would result in them constantly having to wear glasses for reading and near tasks. I see a fair number of referrals who were -1 D or -1.25 D, who had beautifully performed surgery and are now in their 40s or early 50s, and are unhappy because they lost their ability to see at intermediate and near.
Unfortunately, many patients who are interested in refractive surgery think that it’s a panacea that’s going to correct their vision at all distances, and are surprised when you explain that it won’t. These are good cases in which to try monovision if they haven’t already. These concepts are less of an issue when you are dealing with a patient who wears contact lenses on a regular basis, because he understands what life after refractive surgery will be like.
Another patient to be somewhat cautious of is someone who’s unhappy with her glasses, doesn’t like soft lenses, and feels that the only corrective lens remotely adequate for her is a rigid gas permeable contact lens. She’ll often bring in several pairs of glasses with small, 0.25-D differences in sphere or cylinder and declare that none of them work for her. This is another type of patient who’s likely to be unhappy with the results of laser vision correction, and you probably won’t be able to satisfy her.
• Prep the surface. If the patient has significant dry eye, you have to address it. This can take the form of more frequent lubrication, Restasis or Xiidra, or a short course of topical steroids. Also, be sure to treat any external disease like meibomian gland dysfunction and blepharitis with lid hygiene, warm compresses and/or a brief course of steroids, as these conditions can lead to a sterile inflammatory response at the edge of the flap if they’re not addressed prior to refractive surgery.
• Femtosecond flap issues. Though there are several systems with which to make flaps, I routinely use the IntraLase and the Visumax. Each system has its own strengths and weaknesses.
When performing a treatment with the IntraLase, a suction break is very uncommon due to its very strong suction. However, you can still take some extra precautions to ensure this doesn’t occur. First, make sure the patient is relaxed and knows what to expect before you activate the suction. Before you activate it, explain that he’ll feel pressure and the lights will dim or brown out. Have him avoid clenching his facial muscles and/or trying to squeeze out the ring. It often helps to tell patients to keep their shoulders relaxed and to not clench their teeth. Also, explain how long the process will take.
When using the IntraLase, if the patient has a small cornea or a cornea with neovascularization, intracameral bubbles can occur if the photodisruption is very near the limbus. Because of this, you may want to consider creating a smaller-diameter flap, trying to avoid the limbal blood vessels, because the gas can tract back through Schlemm’s canal, go inside the eye, and make eye-tracking with the laser challenging.
With the Visumax laser, instead of suction being applied to the sclera, it gets affixed to the cornea. It’s a very light suction, so it’s more prone to suction breaks. The benefit, however, is that patients get no postop subconjunctival hemorrhage, no discomfort during the treatment and their vision doesn’t go dark during the procedure as it does during IntraLase flap creation. The disadvantage is, if the patient has a strong Bell’s phenomenon or squeezes his eye, he can break suction during the actual treatment.
Because of the different kind of suction, the Visumax calls for a different technique. Once the suction ring is applied to the patient’s eye, he is instructed to look at a green light, which will remain in view throughout the treatment. When the applanation cone is brought down onto the cornea, that green light becomes very clear. At that point, you want to make sure the light is properly centered and then engage suction. When you engage the suction, be sure to tell the patient that it’s all locked in place and that, after about 14 seconds, the green light will get fuzzy, so he should avoid the impulse to chase it—it’s best to look beyond it at that point. A 9-mm flap takes about 18 seconds to create, with the treatment starting in the periphery and then moving to the center. As this occurs, we’ll do a treatment countdown, so the patient can focus on staying still, secure in the knowledge that it will be over at the end of the countdown. We also tell patients that, once the treatment starts, they shouldn’t try to talk, because it can cause their head to move and break suction with a device with relatively light suction like the Visumax.
One tactic I use to try to avoid intraoperative suction breaks is to put my hand on the patient’s head, with my finger on the upper lid to try and detect any attempt at squeezing the eye. The touch of my hand helps calm the patient, but the finger also alerts me if she is squeezing too hard.
If a suction break occurs with the Visumax, the company recommends you re-engage with the same patient interface. Basically, Zeiss says you don’t have to abort the procedure, and you can re-cut at any point. However, if you’ve performed the complete lamellar cut but not the side cut, you have to use a workaround: You need to program the laser to perform another flap. Then, pinch the suction tube with your fingers to simulate suction without the suction ring on the eye. Depress the foot pedal for 13 seconds to discharge the lamellar cut while pinching the tube–essentially wasting the lamellar cut portion of the procedure. After the 13 seconds, you release suction. The system will then detect a suction break. You then apply the suction ring to the eye and complete the treatment with the remaining five seconds. The last five seconds is the side-cutting portion of the treatment. (If you have a suction break after the lamellar cut has been made during LASIK with the IntraLase, you can program the laser to perform a side cut only.)
Tissue bridges can be somewhat challenging to break when you try to lift a Visumax flap in the presence of an opaque bubble layer, which is a film composed of bubbles in the intrastromal interface that can block laser pulses and result in hard-to-lift flaps. The best approach is to try and avoid OBL to begin with. One way to do this is to have Zeiss’ corporate field representatives slightly lower the power so as to not get too much OBL. If the treatment does result in OBL, gently work at the OBL with the instrument when you go to lift the flap, taking great care not to perforate or tear the flap. Additionally, with the Visumax, I’ll typically score the 270-degree side cut first, because occasionally there are tissue bridges not completely cut by the laser. However, if you trace around 270 degrees first, it makes lifting the flap easier because there won’t be tags at the flap edge. Overall, I’ve performed a couple thousand LASIK procedures using the Visumax, and have had only one suction break during flap creation. I re-did it without a problem. I’ve performed more than 20,000 LASIK surgeries with the IntraLase, and have had only one, maybe two, suction breaks. I re-engaged it without a problem in those instances.
On the IntraLase, if you do encounter intracameral bubbles—and there aren’t a lot of them—you can turn down the light source on the excimer to help it track the pupil more accurately. As long as the excimer is capturing the pupil’s edge, you can safely perform the treatment. If, for some reason, the tracker on the Visx excimer laser won’t engage, you can turn it off and do the procedure. Alternatively, you can wait, have the patient come back the following day, and complete the treatment at that time.
If there’s scarring present, such as from an old pterygium that you don’t want to cut through when you’re making the flap, you can program the femtosecond laser to put the hinge in a better place. For instance, if the pterygium scar is nasally located, you can place the hinge nasally. Or, if there’s significant pannus superiorly, make a superior hinge.
Be sure to use surgical drapes that cover the patient’s lashes. If you don’t, the oil from the meibomian glands and debris from the lid margin start to float around the cornea during LASIK, potentially flowing beneath the flap and leading to diffuse lamellar keratitis or, rarely, an infection. Also, if there are a lot of secretions, irrigate them away before you lift the flap, and make sure the fornices are dry while you do the treatment. Otherwise, all that debris has the potential to get under the flap when you reposition it in place. A clean interface not only looks good, but it also lowers the risk of inflammation and infection.
LASIK Complications
Unfortunately, any patient can develop a complication. Though you can’t reduce the rate of complications to zero, you can minimize it.
• Buttonhole flaps. If you’re doing thin-flap LASIK with a femtosecond laser in a patient with a previous scar and you get vertical gas breakthrough, or you get a buttonhole while using a microkeratome, don’t lift the flap and ablate. Instead, stop and replace the flap. Then, let it heal and, two to three months later, perform a surface ablation. It may take a little extra time, and a different procedure, but patients will see well in the end. However, if you lift the buttonholed flap and ablate the cornea during the initial flap creation, the patient will almost certainly get epithelial ingrowth due to the potential mismatch between the flap and the underlying surface, which is more difficult to deal with. Also, epithelial ingrowth in the setting of a buttonhole is a common occurrence and can be difficult to manage.
• Epithelial ingrowth. By way of a quick review, epithelial ingrowth is as its name implies, epithelial cells that grow into the LASIK interface rather than just healing over on the surface of the cornea. There are a couple courses of action you can take in these cases.
We’ve had success with cleaning out the ingrowth and then applying a tissue adhesive to seal down the flap: We published a case series of this approach using Tisseal glue, which we found works well. We’ve also just submitted a case report on a patient in whom we used ReSure sealant on a very large buttonhole that had massive epithelial ingrowth. In some cases, we would be forced to amputate the flap and the patient would develop corneal scarring. Now, however, with the advent of tissue glue and sealant, we can more readily manage these cases.
For cases of LASIK enhancements, we’re beginning to study the prophylactic use of tissue sealant. For instance, if a patient who had LASIK three to five years ago comes in for a touch-up, we’ll perform the ablation and then apply sealant prophylactically on the edge of the flap. In a study of this technique that we’re conducting, in the small number of patients on whom we’ve done this procedure so far, none have had developed epithelial ingrowth. However, it’s worth noting that many surgeons have switched over to surface ablation for these late LASIK enhancements. The problem with that approach, however, is that the patients have a long recovery time and often have a significant hyperopic shift over the first couple of months postoperatively. They’re also a little less satisfied with PRK after having experienced the “wow” factor of LASIK.
• Corneal striae and folds. Due to the more-frequent use of femtosecond lasers, which results in more-uniform flaps than those we used to make mechanically, we don’t encounter striae and flap folds as much as we used to. You can run into this complication, however.
If you do see striae, either micro or macro, it’s important to address them early. If you leave them in place, they become fixed folds that are very difficult to remove. For micro-folds, you can often just smooth them out and eliminate them that way.
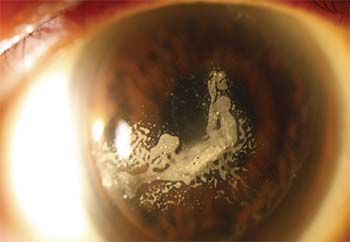 |
| Once ingrowth is removed, recurrence can be prevented by sealing down the flap. |
To smooth out a cornea with a fold, if it’s the first day postop, I’ll take two Weck-Cel sponges and, starting in the center of the cornea, move them in opposite directions across the cornea to stretch it out. This will flatten many small striae.
For fixed folds that have been present for weeks or months, I’ll take the patient back to the LASIK suite and remove the epithelium under topical anesthesia in order to see where the folds are in Descemet’s. This step is necessary because the epithelium can mask the extent of striae. Then, I’ll smooth out the cornea and place sutures that will leave the flap on-stretch. I typically leave the sutures in for three to six weeks depending on the severity of the striae and the length of time the striae have been present.
• Diffuse lamellar keratitis. DLK isn’t that common, but early recognition is still imperative. If you find DLK early on, you can just increase the steroid drops. For example, if I diagnose DLK on postoperative day one, I’ll use Durezol as often as six to eight times per day for a few days and it will effectively resolve it. Using this regimen, I haven’t had anyone go on to stage-IV DLK.
• Interface fluid. This is a rare postop presentation that can occur if a patient is treated with an aggressive course of topical steroids for too long a duration, which can occur if a surgeon believes he’s treating DLK. DLK isn’t chronic, and if a patient has been treated for two weeks with aggressive steroids for DLK, it probably isn’t actually DLK. What can occur instead, however, is a pressure rise from the steroids, causing fluid to accumulate in the interface and creating a “false” anterior chamber effect. Because of this, applanation tonometry in the central cornea will read normal or low, but a device such as the Tonopen, when used in the periphery, will read the actual pressure—often as high as 40 to 80 mmHg. The upshot is that you shouldn’t be using aggressive steroids for more than seven to 10 days for DLK management. A slit lamp exam or anterior segment OCT can reveal this condition or rule it out.
PRK
Once thought to be a phased-out procedure whose time had passed, the advent of LASIK-induced ectasia several years ago brought surgeons back around to the understanding that there are some patients for whom surface ablation is the better choice. Here’s what I’ve learned about it over the years.
• Prep the surface. As with LASIK, you want to make sure that the corneal surface is optimized preop. If the patient has dry eye, be proactive in fitting punctal plugs, either permanent or dissolvable collagen varieties. I’ve found that, if the eye is well lubricated ahead of time, it heals much faster than if it’s a chronic dry eye. Along these lines, if the patient is a smoker, have him stop beforehand, since I feel it can slow epithelial recovery and increase the risk of scarring and haze.
• Epithelial debridement. Though there are several different ways to debride the epithelium, I’ve come to prefer the use of a rotating brush. The brush is very fast, leaves smooth edges and, with practice, you can remove just the right amount of tissue with it. The rotating brush also takes only a few seconds to use, and the debridement is very consistent from eye to eye.
I’ve never been a fan of alcohol-assisted removal because alcohol is a desiccant, and if it’s left on the epithelium too long or spills onto the limbus, it’s very
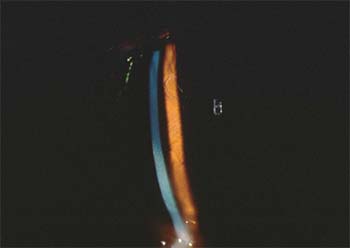 |
| Diffuse lamellar keratitis, if caught early, can be treated with increased steroids. |
• Mitomycin C. Besides postop pain, haze and regression are the next serious concerns. To help stave them off, I use MMC in virtually all cases. I’ll vary the exposure time of the 0.02% MMC based on the amount of tissue removal. If it’s more than 90 to 100 µm, I’ll use it for 40 seconds. For 45 to 89 µm, I’ll apply it for 25 seconds, and if the correction is under 45 µm, I’ll use it for 15 seconds. When we use it in this way, the risk of developing scarring or haze is significantly less than 1 percent and, if the patients do develop scarring or haze, it tends to be relatively mild and treatable.
• Pain management/postop surface issues. As alluded to above, postop pain is a consideration with PRK. Though we can’t eliminate it, we can take some steps to minimize it. We use chilled BSS during the procedure, and have patients put their lubricant drops in the fridge at home when they’re not using them. My postop regimen consists of the following:
• one NSAID drop after the bandage contact lens is in place (I prefer an 8.4 base-curve Acuvue);
• a fourth-generation fluoroquinolone and Pred Forte until the epithelium heals;
• following re-epithelialization, Pred Forte q.i.d. for two weeks, then b.i.d. for two weeks (for lower corrections, possibly q.i.d. for 10 days and b.i.d. for 10);
• avoid postop topical NSAIDs, which I feel don’t offer a lot of comfort and can slow down re-epithelialization.
Also, before surgery, we’ll have patients begin taking ibuprofen to try to get ahead of the pain cycle and circumvent the inflammation cycle. We’ll also occasionally give Tylenol with codeine or Vicodin for breakthrough pain.
In cases of lid swelling postop (the eye itself doesn’t discriminate between the eye and lid in cases of inflammation), we’ll recommend an ice pack.
With regard to the ocular surface, some patients will develop transient dry eye postop. In such cases, we’re aggressive with lubricants. If that’s not enough, we’re quick to use Restasis or Xiidra. If those aren’t effective enough, we have a low threshold for going to plugs because we’ve found that patients with symptomatic dryness preop really respond well to plugs postop.
Though refractive surgery has been around a while and it may seem that some surgeons have gotten it down to a science, a tough case will surprise you sooner or later. I hope that these tips and strategies for lamellar and surface procedures will help make it much later. REVIEW
Dr. Manche is the director of cornea and refractive surgery at the Stanford University Eye Laser Center, and a professor of ophthalmology at the university. He is a consultant for Allergan, Avedro, Shire, J & J Vision, Carl Zeiss Meditec, Ocular Therapeutix and Avellino Labs.
1. He L, Manche EE. Fibrin glue for prevention of recurrent epithelial ingrowth under a LASIK flap with a central buttonhole defect. J Cataract Refract Surg 2012;38:10:1857-60.