Our differential diagnosis included infectious etiologies such as endophthalmitis, with endogenous endophthalmitis
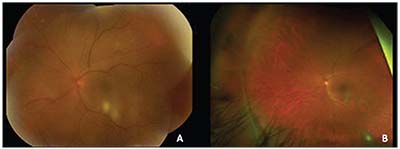 |
| Figure 3. (A) Color fundus photograph of the left eye two months after initiation of antifungal therapy. (B) Wide-field color fundus photo of the left eye three months after initiation of antifungal therapy. |
For further diagnostic clarity, our patient underwent intravitreal tap and injection with voriconazole 1,000 mcg/0.1 ml. She was empirically started on voriconazole 200 mg twice daily. Her anterior uveitis was treated with prednisolone acetate 1% every two hours in the left eye. Unfortunately, the cultures taken had no yield.
Over the next three months, her visual acuity in the left eye improved from count fingers to 20/80 while on antifungal therapy. Her examination showed resolving anterior chamber, vitreous and chorioretinal inflammation (Figure 3). She was subsequently tapered off prednisolone acetate. However, at her next visit, her vision in the left eye had deteriorated to 20/200. Optical coherence tomography and fluorescein angiography showed interval development of cystoid macular edema in the left eye (Figure 4). She underwent injection of sub-Tenon’s triamcinolone acetonide. Upon follow-up, her vision improved to 20/70, though her CME persisted, for which she was next treated with intravitreal bevacizumab 1.25 mg. Given full resolution of her panuveitis, her voriconazole was discontinued, and her diagnosis was consistent with presumed fungal endogenous endophthalmitis. Unfortunately, her macular edema and epiretinal membrane persisted, requiring a pars plana vitrectomy and epiretinal membrane peel.
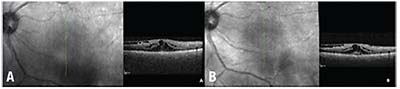 |
| Figure 4. Optical coherence tomography of the left eye before (A) and after (B) sub-Tenon’s triamcinolone acetonide. |
Discussion
Fungal endogenous endophthalmitis, caused by hematogenous seeding of the choroid with subsequent posterior and anterior segment involvement, can lead to devastating visual outcomes. The predominant microorganisms implicated are Candida and Aspergillus species. Patients with a relative immunosuppression from diabetes, liver disease, renal failure, malignancy, organ transplantation or HIV are at risk for developing this infection, as are those undergoing instrumentation of the GI tract or abdominal surgery, those with indwelling catheters, and intravenous drug users.1
As in our case, which was ultimately culture-negative, the cornerstone of diagnosis remains clinical suspicion, given the abrupt onset following complicated colonoscopy, examination with classic findings like “string of pearls” vitritis and yellow-white chorioretinitis, and response to empiric treatment. Fungal culture via intravitreal tap is often low-yield, as is anterior chamber paracentesis, compared to diagnostic vitrectomy.2
Once fungal endogenous endophthalmitis is suspected, empiric treatment with antifungals should be initiated. Prior to the development of voriconazole, intravitreal amphotericin B with or without pars plana vitrectomy was considered standard-of-care. However, systemic voriconazole, introduced in 2002, has excellent bioavailability and less retinal toxicity. Thus, systemic treatment with or without intravitreal voriconazole is more frequently used.3 While no randomized controlled trial has been performed regarding the efficacy of pars plana vitrectomy in fungal endogenous endophthalmitis, common indications for vitrectomy include diagnostic vitrectomy, non-clearing inflammatory vitreous debris, uncontrolled infection despite medical management, and release of vitreoretinal traction to treat retinal detachments and other structural sequelae.1,3 Some recent case series suggest improved outcomes with immediate total vitrectomy.4,5
Another sequela seen in our patient was the development of epiretinal membrane.6 Though literature surrounding the specific treatment of epiretinal membrane after fungal endophthalmitis is sparse, small series of patients with epiretinal membrane-induced tractional retinal detachments achieved good structural outcomes after pars plana vitrecomy and membrane peeling.7,8
The largest contributor to our patient’s poor visual outcome was cystoid macular edema. Periocular, intravitreal, implanted and systemic steroids have been shown to be effective in the control of uveitic macular edema,9 but must be used with caution in patients with infectious uveitis and fungal endophthalmitis. A case series from L. V. Prasad Eye Institute in India showed improvement in inflammation in patients with fungal endophthalmitis concomitantly given antifungals and steroids, without clear evidence of reactivation of the infection.10 Steroids may be beneficial in infectious uveitic macular edema as well—in a series of eight patients, intravitreal dexamethasone implants showed improvement in visual acuity and resolution of edema without reactivation of infection.11 There have only been a few small studies evaluating the use of anti-VEGF agents such as bevacizumab for the treatment of uveitic macular edema. Anti-VEGF agents may prove to be a successful and safer alternative for treatment of infectious and non-infectious uveitic macular edema for those at risk of increased intraocular pressure and with steroid refractory macular edema.12-15 Randomized, controlled trials are underway examining the use of different approaches, including intravitreal ranibizumab, periocular steroid injections, intravitreal methotrexate and intravitreal steroid injections and implants.16-18 REVIEW
1. Lingappan A, Wykoff CC, Albini TA, et al. Endogenous fungal endophthalmitis: Causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012;153:1:162-6.e1.
2. Liu K, Fang F, Li H. Reliability of vitreous histological detection of pathogenic fungi in the diagnosis of fungal endophthalmitis. Eye (Lond) 2015;29:3:424-7.
3. Chee YE, Eliott D. The role of vitrectomy in the management of fungal endophthalmitis. Semin Ophthalmol 2017;32:1:29-35.
4. Behera UC, Budhwani M, Das T, et al. Role of early vitrectomy in the treatment of fungal endophthalmitis. Retina 2017; May 23. [Epub ahead of print]
5. Birnbaum FA, Gupta G. The role of early vitrectomy in the treatment of fungal endogenous endophthalmitis. Retin Cases Brief Rep 2016;10:3:232-5.
6. Smiddy WE. Treatment outcomes of endogenous fungal endophthalmitis. Curr Opin Ophthalmol 1998;9:3:66-70.
7. Naoi N, Sawada A. Effect of vitrectomy on epiretinal membranes after endogenous fungal endophthalmitis. Jpn J Ophthalmol 1996;40:3:434-8.
8. McDonald HR, De Bustros S, Sipperley JO. Vitrectomy for epiretinal membrane with Candida chorioretinitis. Ophthalmology 1990;97:4:466-9.
9. Karim R, Sykakis E, Lightman S, Fraser-Bell S. Interventions for the treatment of uveitic macular edema: A systematic review and meta-analysis. Clin Ophthalmol 2013;7:1109-44.
10. Majji AB, Jalali S, Das T, Gopinathan U. Role of intravitreal dexamethasone in exogenous fungal endophthalmitis. Eye (Lond) 1999;13(Pt 5):660-5.
11. Fonollosa A, Llorenc V, Artaraz J, et al. Safety and efficacy of intravitreal dexamethasone implants in the management of macular edema secondary to infectious uveitis. Retina 2016;36:9:1778-85.
12. Al-Dhibi H, Hamade IH, Al-Halafi A, et al. The effects of intravitreal bevacizumab in infectious and noninfectious uveitic macular edema. J Ophthalmol 2014;2014:729465.
13. Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-Tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina 2011;31:1:111-8.
14. Cervantes-Castaneda RA, Giuliari GP, Gallagher MJ, et al. Intravitreal bevacizumab in refractory uveitic macular edema: One-year follow-up. Eur J Ophthalmol 2009;19:4:622-9.
15. Cordero Coma M, Sobrin L, Onal S, et al. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology 2007;114:8:1574-9.e1.
16. The LIMO study: Lucentis for treatment of uveitic patients with refractory cystoid macular oedema. ClinicalTrialsgov Identifier: NCT01564108: Moorfields Eye Hospital NHS Foundation Trust.
17. PeriOcular and INTravitreal Corticosteroids for Uveitic Macular Edema Trial (POINT). ClinicalTrials.gov Identifier: NCT02374060: JHSPH Center for Clinical Trials and National Eye Institute (NEI).
18. Macular Edema Ranibizumab v. Intravitreal Anti-inflammatory Therapy Trial (MERIT). ClinicalTrials.gov Identifier: NCT02623426: JHSPH Center for Clinical Trials and National Eye Institute (NEI).



