 |
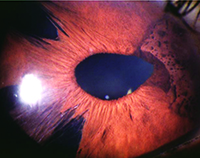
| In addition to corneal edema, ICE syndrome is commonly associated with iris abnormalities, including atrophy, stretch holes, nodules and pupillary distortion, and the incidence of secondary glaucoma is high. |
The incidence of secondary glaucoma in patients with ICE syndrome is fairly high, and its management can be very challenging; it’s often accompanied by corneal edema and requires multiple surgeries. Generally, ICE syndrome occurs unilaterally, and it’s commonly associated with corneal edema and iris abnormalities, including atrophy, stretch holes, nodules and pupillary distortion. Affected patients tend to be young, with no known comorbidities.
Glaucoma associated with ICE syndrome is recalcitrant and often difficult to control. As a result, modification of standard surgical techniques is necessary to optimize outcomes. Here, we’d like to offer some strategies that can help you make the best of this challenging condition.
Surgical Options
Because glaucoma secondary to ICE syndrome frequently involves extensive synechial angle closure, medications are often not sufficient to control the intraocular pressure. Likewise, laser trabeculoplasty is often unsuccessful, despite angles that appear open. Laser trabeculoplasty targets the trabecular meshwork cells; in ICE syndrome, the endothelial membrane or peripheral anterior synechiae preclude access to the meshwork cells. Transscleral cyclophotocoagulation can be effective, although it doesn’t address the primary outflow obstruction. We tend to reserve it for end-stage disease and for patients who don’t want or can’t have incisional surgery.
Because medicinal therapy is often inadequate and the utility of laser is limited, surgical procedures tend to be the treatment of choice. However, the newer Schlemm’s canal-based procedures such as Trabectome and iStent are not feasible in most ICE syndrome patients, and they are unlikely to achieve lasting results even when areas of open-appearing angle exist, due to continued endothelial membrane proliferation. Procedures that bypass the trabecular outflow pathway altogether, such as trabeculectomy with mitomycin-C and aqueous shunt surgery, are considerations. Of these, we favor tube shunts over trabeculectomy, based on our personal experience and the limited data in the published literature.
What the Data Shows
To compare the potential value of these procedures, it’s worth looking at the data reported in the literature. One study published in 2000 reported the outcomes of trabeculectomy with mitomycin-C performed on 10 ICE syndrome patients.1 At a mean follow-up of 14 months, the success rate—defined as an IOP less than 21 mmHg—was 80 percent. However, this follow-up period was short by glaucoma standards. For comparison, another study published in 2001 reported the results of trabeculectomy performed with either mitomycin-C or 5-fluorouracil on 12 ICE syndrome patients; at five years, using the same criteria, the success rate was only 29 percent.2 (Of course, it’s not uncommon to have an increasing number of patients fail after trabeculectomy the longer you follow them, but ICE syndrome patients tend to have a much greater failure rate than standard open-angle glaucoma patients would have.)
ICE syndrome patients have a number of risk factors that can lead to failure of trabeculectomy. They tend to be younger than the average glaucoma patient, which means they have more robust healing and may scar more easily; the proliferating membrane associated with ICE syndrome may block the internal ostium; many of these patients have had prior surgeries, which can add to their risk of failure; and these patients often need other surgeries in tandem with or subsequent to the glaucoma surgery, such as cataract surgery or corneal transplantation. That combination can put even a well-functioning filter at risk.
Aqueous shunts have also demonstrated limited success in ICE syndrome patients, but they tend to do better than trabeculectomies. A 1999 study in which 10 ICE syndrome patients underwent tube implantation had a five-year success rate of 75 percent (although tube revision or repositioning was required in three of the patients).3 Another study published in 2001 followed 21 patients who received tube shunts; at five years their success rate was 53 percent.2 While these were retrospective studies, there is reason to believe that the long-term success rate of tube implant surgery is superior to that of trabeculectomy.
When shunts fail in ICE syndrome, they can do so for a variety of reasons. For example, the capsule can become excessively thick. (This can happen in any tube-shunt patient, but the tendency for this to occur may be greater in ICE syndrome patients than in patients with other types of glaucoma.) Also, the proximal tube ostium can become blocked, either by the endothelial membrane or by the iris, because of progressive anterior synechiae formation. This accounts for the need to reposition tubes in some patients; if the iris gets pulled up into the tube, for example, the tube may become occluded and need to be repositioned into the ciliary sulcus or the pars plana. Leaving the tube long so that it extends far away from the anterior chamber angle and the iris can often prevent these issues.
To summarize, tubes often work better in ICE syndrome patients because they tend to be more resilient than trabeculectomy; they are less prone to failure due to subconjunctival or episcleral scarring (which is especially important given the younger age of these patients); they are less affected by inflammation; and they are less affected by prior, concurrent or subsequent surgery. All of these things are common concerns in ICE syndrome patients.
Assessing the Eye
Clearly, in ICE syndrome patients, the location of tube placement is critical. The standard choices for placement would be the anterior chamber, the ciliary sulcus, or through the pars plana into the anterior vitreous cavity. The surgical approach that makes the most sense will vary.
Preoperative assessment is always critical. Perform gonioscopy to look for the location and height of the peripheral anterior synechiae. Locating the synechiae can help to guide your tube placement to locations where the angle is still open. (Although the membrane of proliferating cells can be visualized on pathologic specimens, it’s translucent and can’t be visualized clinically.)
If the patient is phakic, note the status of the lens. Does the patient have a cataract, or is the lens clear? If the patient is pseudophakic, note the type of lens and the status of the posterior capsule. (If the patient is aphakic, that presents different options and needs; we’ll discuss those shortly.)
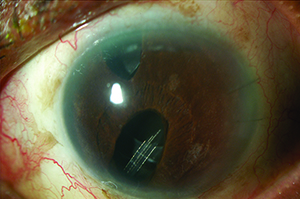 | 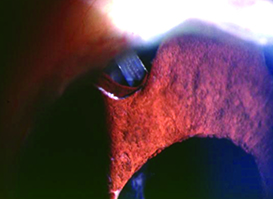 | |
| Choice of tube location in the prescence of ICE syndrome is critical to preventing the tube from becoming occluded by proliferating endothelial cells. One option is to place the tube so that the tip lies within a preexisting iris defect, as shown above. | ||
If the patient does have corneal edema, you may need to perform a corneal transplant, Descemet’s stripping automated endothelial keratoplasty or Descemet’s membrane endothelial keratoplasty. In any case, you have to be able to see through the cornea well enough to do the glaucoma surgery and any other concomitant surgery.
It’s also important to assess pupil size and configuration, and the location of any vitreous, particularly in aphakic or pseudophakic patients. If vitreous is present in an area that might allow it to sneak into the tube tip postoperatively, perform a vitrectomy and remove that vitreous at the time of tube placement.
Placement of the Tube
If the cornea is clear and the anterior chamber angle is open (as it might be in a Chandler’s syndrome patient) with no visible synechiae, the tube can be positioned in the anterior chamber. While leaving the tube tip long can help prevent occlusion, it’s also important in the event that the tube needs to be repositioned to the sulcus or pars plana at a future time. Creating a curved course for the extraocular portion of the tube, between the endplate and the insertion site, will also allow a little extra tube length should you need it for future repositioning.
If concurrent cataract surgery is being performed, the tube can be positioned in the anterior chamber, the ciliary sulcus or the pars plana. Ciliary sulcus or pars plana placement is generally preferred in pseudophakic and aphakic patients, as a way to decrease the potential risk of corneal decompensation requiring transplantation. However, in order to put the tube through the pars plana and into the anterior vitreous cavity, a concurrent vitrectomy is required, adding more surgery to your procedure.
In ICE patients, it’s especially important to maximize the distance between the tip of the tube and the posterior surface of an already compromised cornea. Additionally, as mentioned earlier, longer tube length can minimize the potential for occlusion of the proximal ostium by iris or endothelial membrane.
Regarding iris contact, we agree with some surgeons that resting on the iris is preferable to touching the cornea, but in ICE syndrome patients we think it’s equally important that the tube not touch the iris because of the underlying disease process. The synechiae tend to pull the iris forward, toward the angle and the cornea. Contact between the tube and the iris may accelerate synechiae formation. So, if the tube is too deep in the anterior chamber, the iris will get pulled up and potentially occlude the tube. In addition, the membrane on the surface of the iris can overgrow the proximal tube tip; contact between the iris and tube tip may facilitate that overgrowth. So ideally you should make sure the tube tip is close to, but above the surface of the iris. (Or, as mentioned earlier, it can be positioned within an iris defect or iridectomy when one is present in an appropriate location, to minimize the risk of occlusion.)
Sometimes the iris may have an existing stretch hole or surgical iridectomy. In that situation you can position the tube within the iridectomy so that it’s far away from the cornea and not on top of the iris, minimizing the risk of occlusion. Fortunately, silicone is a poor substrate for membrane growth, so the membrane will only block the tube if the opening is contacting the iris or immediately adjacent to it.
Diffuse synechiae may preclude anterior chamber placement and require placement in the sulcus or pars plana. In fact, ciliary sulcus placement is often facilitated when the iris is pulled forward by synechiae, enlarging the sulcus space. However, insertion of the tube into the ciliary sulcus or anterior vitreous cavity necessitates removal of the lens, whether it’s clear or not.
We believe that it’s important to do a complete pars plana vitrectomy in conjunction with pars plana tube placement, as opposed to a limited anterior vitrectomy (even if the patient doesn’t have ICE syndrome), because any residual vitreous will eventually find its way into the tube opening and block it.
Multiple Surgeries
In addition to poorly controlled intraocular pressure, some ICE syndrome patients will have corneal edema that requires a corneal transplant. If you need to do more than one surgery, such as a corneal transplant in addition to the glaucoma surgery—and possibly cataract surgery and a vitrectomy as well—the timing of the surgeries may depend on the extent of the corneal edema and the degree to which it obscures the surgeon’s view into the eye.
If the patient has early corneal edema, we prefer to stage the surgeries. As long as the view is good enough to perform the surgery, we’ll do the shunt first, sometimes combining it with cataract removal or vitrectomy so we can put the tube through the pars plana. After three or four months, when the pressure is stable and the inflammation has subsided, we’ll go back and do the corneal transplant.
This is a good approach because the endothelium of the corneal graft is delicate and may be adversely affected by excessive intraocular inflammation, variable intraocular pressures and potential early postoperative complications, all of which can be associated with combined surgery. On the other hand, graft survival can be enhanced by performing corneal transplantation alone in an eye that is quiet with a stable and well-controlled intraocular pressure several months after aqueous shunt surgery. Fortunately, most of these patients have a healthy, well-seeing fellow eye, so rapid visual rehabilitation of the eye with ICE syndrome is less of a concern.
Of course, in some cases you can’t see well enough to do any intraocular surgery because the corneal edema is substantial. If that’s the situation, you may need to do the surgeries concurrently—first, do the corneal transplant; then perform a vitrectomy, remove the cataract (if the patient is still phakic) and implant the tube in the sulcus or pars plana. Our bias is to place the tube in the pars plana in this situation, but that’s just our preference; others prefer to put the tube in the sulcus.
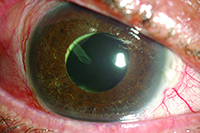 |
| In many pseudophakic patients, the tube can be placed within the ciliary sulcus, as seen above. If the patient is phakic, the lens must be removed prior to placement in the sulcus or anterior vitreous cavity. |
Hoping for a Cure
Of course, secondary glaucoma doesn’t occur in every ICE syndrome patient. However, even those who don’t develop secondary glaucoma may still develop corneal edema and require a corneal transplant for visual rehabilitation. Meanwhile, when glaucoma does develop because of angle closure, managing it appropriately can be crucial to maintaining the patient’s vision.
Ideally, we’d like to be able to address the underlying cause of the ICE syndrome by correcting the endothelial cell abnormality and preventing these cells from proliferating. Resolving the problem at the cellular level would provide a true long-term solution for these patients and eliminate the problem of secondary glaucoma. It is our hope that this will happen at some point in the near future. REVIEW
Dr. Sidoti is a professor of ophthalmology and deputy chair for clinical affairs in the department of ophthalmology at New York Eye and Ear Infirmary of Mount Sinai and the Icahn School of Medicine at Mount Sinai in New York City. He has previously received speaking honoraria from NeoMedix. Dr. Schulhof is a glaucoma fellow in the department of ophthalmology at New York Eye and Ear Infirmary of Mount Sinai and the Icahn School of Medicine at Mount Sinai in New York City.
1. Lanzl IM, Wilson RP, Dudley D, Augsburger JJ, Aslanides IM, Spaeth GL. Outcome of trabeculectomy with mitomycin-C in the iridocorneal endothelial syndrome. Ophthalmology 2000;107:295-297.
2. Doe EA, Budenz DL, Gedde SJ, Imami NR. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology 2001;108:1789-1795.
3. Kim DK, Aslanides IM, Schmidt CM, Spaeth GL, Wilson RP, Augsburger JJ. Long-term outcome of aqueous shunt surgery in ten patients with iridocorneal endothelial syndrome. Ophthalmology 1999;106:1030-1034.



