Intranasal nasolacrimal duct cysts are nasal mucosal cysts emanating from under the inferior turbinate. They result from the accumulation of nasolacrimal secretions into blunted mucosal tissue at the level of an imperforate valve of Hasner. Although they are usually associated with dacryoceles or dacryocystitis in infancy, they can occur in infants with varying symptoms of nasolacrimal duct obstruction.1,2 In this review we’ll examine the embryological origin of these cysts, their epidemiology, as well as the diagnostic criteria and management options. We’ll also discuss the pros and cons of new surgical techniques and devices used in the management of these intranasal cysts.
Embryology and Pathogenesis
As the 5-to-6-week-old embryo develops in utero, the lateral nasal and maxillary prominences fuse and entrap a double layer of epithelial cells, which later undergoes canalization between the eighth week of gestation and birth to form the duct.3 Incomplete canalization leads to nasolacrimal duct obstruction at the level where the canalization process failed to occur, and the obliteration can be purely membranous (mucosal soft tissue) or may have an osseous component (agenesis of the distal nasolacrimal duct). In fact, an autopsy of 15 stillborn infants demonstrated that 73 percent of newborns had imperforate nasolacrimal ducts.4 Attempts at injecting fluid into the upper and lower canaliculi resulted in ballooning of mucosal tissue under the inferior turbinate in this study, which clinically resembled the appearance of intranasal cysts.
An imperforate nasolacrimal system will invariably lead to pressure buildup and may lead to backflow of material, resulting in symptoms of nasolacrimal duct obstruction such as tearing or discharge. Alternatively, as the pressure builds up within the nasolacrimal system, ballooning of the lacrimal sac occurs, and a one-way valve effect may result between the lacrimal sac and canaliculi. This is also thought to occur in cases where a common canaliculus is absent, and the upper and lower canaliculi open in the sinus of Maier at an acute angle, preventing the backflow of material through the canaliculi and punctae; this will manifest as a dacryocystocele or dacryocystitis. Intranasal cysts may therefore occur in the setting of symptoms of nasolacrimal duct obstruction, dacryocele or dacryocystitis, unilaterally or bilaterally.5
Background and Epidemiology
The incidence of intranasal cysts is typically observed and reported in the setting of an established diagnosis of dacryocele, dacryocystitis or nasolacrimal duct obstruction. Infantile dacryoceles occur in about 1 to 4 percent of patients with symptoms of nasolacrimal duct obstruction, and acute dacryocystitis occurs in 2 to 3 percent of children with nasolacrimal duct obstruction.1,4,6-9 The average age at presentation for dacryoceles ranges from birth to one to two weeks, with a female preponderance of 63 to 80 percent.10-14 Dacryocystitis may occur anytime from as early as two days after birth to a few months of age.1,12 Evolution
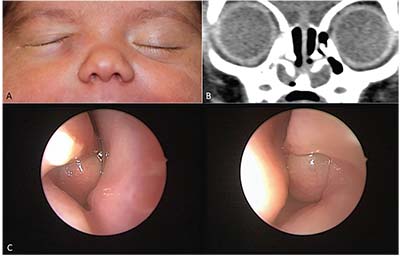 |
| Figure 1. Bilateral intranasal cysts in a patient presenting with a unilateral right dacryocele: A) 1-month-old infant with a right-sided mass below the medial canthus. B) CT of the sinuses with bilateral intranasal cysts below the inferior turbinates. C) Endonasal, endoscopic view of each intranasal cyst below the inferior turbinates. |
from a congenital dacryocele to an acute dacryocystitis may occur in 20 to 72.5 percent of patients.7,10-16
The association between intranasal cysts and nasolacrimal duct obstruction was first noted in 1982.17 It wasn’t recognized until later that infantile dacryocystitis or dacryocystocele are almost invariably associated with findings of an intranasal cyst, according to several studies.1,2 One study found that 23/24 infants (95.8 percent) aged 4 days to 10 weeks with dacryocystoceles had associated intranasal cyst on nasal endoscopy. In another paper, a group of 33 patients (16 with acute dacryocystitis and 17 with dacryocystoceles) was found to have a 100-percent incidence of intranasal cysts. Furthermore, nasolacrimal duct cysts were found in 44 percent of infants younger than 6 months old with severe symptoms of nasolacrimal duct obstruction, and 6 percent of children older than 18 months at the initial time of their probing.1,18
Evaluation and Diagnosis
Infantile dacryocystocele usually occurs in the first few weeks of life, and typically presents as a bluish soft tissue swelling medial and inferior to the medial canthus (Figure 1). Patients should be thoroughly investigated for any signs of infection such as fever, or purulent discharge upon massage of the lesion. In dacryocystitis, however, there might be overt evidence of an infected system such as fever, redness and swelling over the tear sac, and purulent discharge (Figure 2). Occasionally, infants may also have difficulty breathing, especially while feeding. Prompt diagnosis is necessary as neonates are relatively immune-compromised and at risk for sepsis and even meningitis if left untreated. Dacryocystitis may evolve into a lacrimal abscess, so the skin overlying the infected area should be examined for the presence of a fistula.
Other conditions that may mimic the presentation of a dacryocele/dacryocystitis include:
• encephaloceles;
• infantile hemangiomas;
• orbital cellulitis;
• dermoid and epidermoid cysts;
• Zimmerman’s Tumor (phacomatous choristoma);
• venolymphatic malformations (lymphangioma); and
• malignant tumors (neuroblastoma, rhabdomyosarcoma).
Imaging
The diagnosis of infantile dacryocystitis or dacryocystocele is typically a clinical one and does not require imaging. However, in cases such as infants in respiratory distress, in severe and/or rapidly progressive cases, and whenever there is proptosis or globe displacement, imaging can provide a useful adjunct for proper diagnosis, and to rule out other etiologies (venolymphatic malformations, infantile
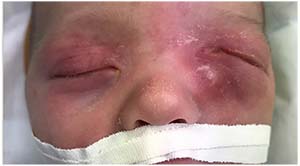 |
| Figure 2. Left-sided dacryocystitis in a patient photographed just before surgery. |
hemangiomas, etc.).10,13,15 Computed tomography and magnetic resonance imaging are helpful in delineating the lacrimal sac, detecting nasolacrimal duct distention and identifying the intranasal cysts (Figure 1B). Imaging is especially helpful in ruling out other potential etiologies of a medial canthal mass such as encephaloceles or vascular malformations. High-frequency ultrasonography has also been suggested as a useful adjunct in the diagnosis of neonatal dacryocele/dacryocystitis that doesn’t require sedation, as opposed to magnetic resonance imaging and computed tomography.19 A subcutaneous heteroechoic cystic lesion without vascular flow is typical of a dacryocele, whereas prominent vascular flow within the lesion on color Doppler is a typical finding in infantile hemangioma. When a component of dacryocystitis is present, peripheral Doppler signal can be detected as a result of inflammation at the level of the abscess wall.
In summary, whenever you suspect a vascular lesion, ultrasonography may help with the diagnosis without exposing the infant to sedation.
Management
Once a diagnosis of a dacryocele or dacryocystitis is made in an infant, proper counseling with the parents about the potential gravity of this condition should follow. Assessment of respiratory distress is paramount as the presence of intranasal cysts can obstruct the nasal airway (Figure 1c). Infants with a dacryocele should be followed closely on an outpatient basis, whereas patients with an established diagnosis of dacryocystitis should be admitted for observation and treatment. An algorithm for the treatment of a mass over the lacrimal sac in an infant is summarized in Table 1.
In general, patients with unilateral dacryoceles without signs of infection can be managed conservatively with attempted massage and antibiotic ointment if there are no signs of respiratory distress, and an in-office probing and irrigation may be successful in treating the condition.
When bilateral dacryoceles are present there is a significant risk for airway obstruction, and it’s highly
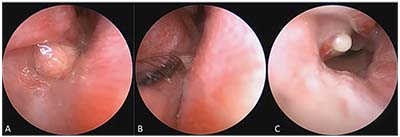 |
| Figure 3. Intranasal cyst treatment with a microdebrider. A) An intranasal cyst associated with the right valve of Hasner is visible under the right inferior turbinate. B) Microdebridement of the cyst. C) Immediately post-microdebridement, mild pressure on the distended lacrimal sac expresses the contents into the intranasal space. |
recommended that the patient be admitted to the hospital with a prompt plan for surgical care. In cases of suspected dacryocystitis, intravenous antibiotics should be added. Strongly consider surgical drainage under direct visualization and collecting the specimens for microbial cultures if there’s no improvement after 24 hours of intravenous antibiotic treatment. If a unilateral dacryocystocele is persistent two weeks after an in-office probing and attempted lacrimal massage, surgical drainage is recommended.
Surgery
Surgery is performed under general anesthesia. First, the nasal cavity is decongested with ¼” cottonoids soaked in a solution of 1 ml of 0.25% oxymetazoline and 1 ml of 1:1,000 (0.1%) epinephrine. Be sure to examine both sides, as a significant proportion of patients with unilateral symptoms have bilateral intranasal cysts. Next, visualize the intranasal cavity with a 0- or 30-degree pediatric endoscope and examine it
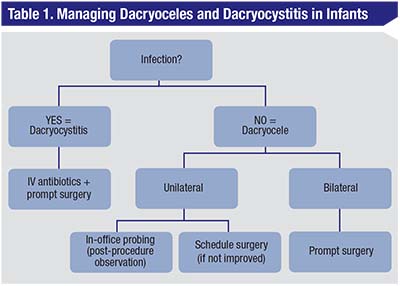 |
| With the presence with erythema (with or without fever) over a mass below the medial canthus, dacryocystitis should be the presumed diagnosis. An infant should be admitted to a hospital and started on IV antibiotics. If there is no improvement over a short course of treatment (24 hours), strongly consider immediate surgery. If you don’t suspect an infection and have a presumed diagnosis of unilateral dacryocele, you can attempt in-office probing. A child must be observed after this procedure for acute respiratory distress and then can be sent home with instructions to massage the tear duct with the application of topical antibiotics. If the dacryocele recurs, consider surgery. In patients with bilateral dacryoceles, don’t attempt in-office probing due to the risk of respiratory distress; instead, consider prompt surgical microdebridement. |
for the presence of nasolacrimal duct cysts before you exert any pressure on the lacrimal sac. Use a Freer elevator where necessary to gently elevate the inferior turbinate and facilitate access to the intranasal space, which is typically tight in infants. Care must be taken to replace the inferior turbinate at the completion of the procedure as failure to do this may cause respiratory compromise, since neonates are obligate nasal breathers. When you identify a cyst, you can apply gentle pressure to the lacrimal sac area; that may reveal bulging of the cyst intranasally.
Though surgeons have described different surgical instruments for the rupture/marsupialization of the cyst—from an alligator forceps or a sickle-cell blade passed through the nostrils, to a Bowman probe passed through the nasal duct and moved in a seesaw fashion to puncture the cyst1,12,20—we recently shifted to using a microdebrider instrument for the purpose of cyst marsupialization (Figure 3). In the following section, we’ll describe how the microdebrider works and the best way to employ it.
Microdebrider-assisted Marsupialization
The modern microdebrider as we know it today was first introduced in 1994 by Stryker for use in endoscopic sinus surgery.21-23 It’s a powered instrument that can simultaneously irrigate, aspirate and remove tissue, leading to decreased operative time, better hemostasis and improved visualization. It consists of three parts: a power unit or console; a handpiece that can be connected to a suction source; and a disposable blade that can be connected to an irrigation source through separate tubing. Available microdebriders include the Diego Elite (Olympus America, Center Valley, Pennsylvania), the ESSx Microdebrider (Stryker, Kalamazoo, Michigan) and the Straightshot M4 (Medtronic, Jacksonville, Florida).
In most microdebriders, the blade sits at the tip of the device when connected to the handpiece and comes in different sizes, designs and shapes. The blade itself consists of two hollow concentric outer and inner cylinders, with the inner cylinder or shaft containing the blade edge that rotates or oscillates within the outer shaft and aligns with the outer shaft’s lumen at the oscillating cycle interval (Figure 4). Suction pulls the tissue into the lumen when the window of the outer shaft is open. The blade of the inner shaft then shears/cuts the tissue as it closes the window/opening, and irrigation and suction help remove the debrided tissue through the inner shaft lumen.21
The blades of the microdebrider are manufactured in different shapes, and those used in sinus surgery are usually angled for better access to the different sinus cavities. For the purpose of intranasal cyst removal, however, we typically use the Straightshot M4 Microdebrider with a 2.9 mm Tricut blade that oscillates at 5,000 RPM (which is the recommended manufacturer’s setting for soft tissue removal).
Since the microdebrider is very effective at removing tissue from the surgical field, you have to make sure not to aspirate all the fluid out of the intranasal cysts. Therefore, gentle use of the microdebrider is recommended, followed by intranasal culturing of the extruded fluid. Although fluid can be recovered from the downstream suction cup for cultures and histopathology, this fluid is diluted with the irrigation fluid: Hence you can get better results from direct culture swabs of the infected fluid.
Once adequate cultures have been acquired, you can accomplish complete marsupialization until there’s an adequate opening in the mucosal tissue while, at the same time, taking care not to injure the inferior turbinate. A 23-gauge cannula can be used to inject fluorescein-diluted solution into the lacrimal system.
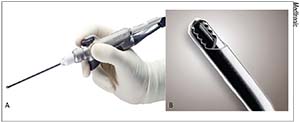 |
| Figure 4. The Straightshot M4 Microdebrider (A) with a 2.9 mm Tricut blade (B) |
Patency is confirmed when the free flow of dye is visualized endoscopically. We prefer to intubate the nasolacrimal system with a monocanalicular stent at the completion of the procedure, which is then removed three months postoperatively. The inferior turbinate is replaced as previously mentioned, and the patient is admitted for postoperative observation.
That being said, the microdebrider is a surgical commodity that might not be readily available or accessible to all surgical centers. Furthermore, its cost may not justify its use in certain cases; hence, we suggest the use of the microdebrider be reserved for select or appropriate cases.
Conclusion
Intranasal cysts result from an imperforate nasolacrimal system and are almost invariably present in infants presenting with dacryoceles and dacryocystitis. Prompt diagnosis and management is critical for optimal care. When conservative measures have failed in the management of dacryoceles, or in cases of imminent or established dacryocystitis, microdebrider-assisted marsupialization represents a safe, time-saving and precise technology for surgical treatment. REVIEW
Dr. Khatib is a consultant ophthalmologist at Clemenceau Medical Center (affiliated with Johns Hopkins Medicine International) in Beirut. Dr. Katowitz is an attending surgeon in the division of ophthalmology at the Children’s Hospital of Philadelphia. They have no financial interest in any product mentioned.
1. Lueder GT. The association of neonatal dacryocystoceles and infantile dacryocystitis with nasolacrimal duct cysts (an American ophthalmological society thesis). Trans Am Ophthalmol Soc 2012; 110:74-93.
2. Levin AV, Wygnanski-Jaffe T, Forte V, et al. Nasal endoscopy in the treatment of congenital lacrimal sac mucoceles. Int J Pediatr Otorhinolaryngol 2003;67:3:255-61.
3. Duke-Elder S, Cook C. Normal and abnormal development. Part1:Embryology. In: Duke-Elder S, ed. System of Ophthalmology, Vol. 3. St. Louis: CV Mosby, 1963:241-245.
4. Cassady JV. Dacryocystitis of infancy: A review of one hundred cases. Arch Ophthalmol 1948;39:4:491-507.
5. Jones LT, Wobig JL. Congenital anomalies of the lacrimal system. In: Surgery of the eyelids and the lacrimal system. Birmingham: Aesculapius Publishing Company, 1976:157-173.
6. Becker F. Zum Membtanosen Verschluss des oberen und unteren ended desTranennasenganges. Klin Monbl Augenheilkd 1938;101:569-570.
7. Shekunov J, Griepentrog GJ, Diehl NN, et al. Prevalence and clinical characteristics of congenital dacryocystocele. J AAPOS 2010;14:417-20.
8. Ffooks OO. Lacrimal abscess in the newborn. Br J Ophthalmol 1961;45:8:562-565.
9. Pollard ZF. Treatment of acute dacryocystitis in neonates. J Pediatr Ophthalmol Strabismus 1991;28:6:341-343.
10. Mansour AM, Cheng KP, Mumma JV, et al. Congenital dacryocele. A collaborative review. Ophthalmology 1991;98:1744-51.
11. Paysse EA, Coats DK, Bernstein JM, et al. Management and complications of congenital dacryocele with concurrent intranasal mucocele. J AAPOS 2000;4:46-53.
12. Becker BB. The treatment of congenital dacryocystocele. Am J Ophthalmol 2006;142:835–8.
13. Ali MJ, Psaltis AJ, Brunworth J, et al. Congenital dacryocele with large intranasal cyst: Efficacy of cruciate marsupialization, adjunctive procedures, and outcomes. Ophthal Plast Reconstr Surg 2014;30:346–51.
14. Shashy RG, Durairaj V, Holmes JM, et al. Congenital dacyrocystocele associated with intranasal cysts: Diagnosis and management. Laryngoscope 2003;113:37–40.
15. Boynton JR, Drucker DN. Distention of the lacrimal sac in neonates. Ophthalmic Surg 1989;20:103–7.
16. Wong RK, VanderVeen DK. Presentation and management of congenital dacryocystocele. Pediatrics 2008;122:e1108–12.
17. Raflo GT, Horton JA, Sprinkle PM. An unusual intranasal anomaly of the lacrimal drainage system. Ophthalmic Surg 1982;13:9:741-744.
18. Lueder GT. Endosopic treatment of intranasal abnormalities associated with nasolacrimal duct obstruction. J AAPOS 2004;8:2:128-32.
19. Vázquez-Osorio I, Hernández-Martín A. Usefulness of ultrasonography in the diagnosis of neonatal dacryocystocele. Pediatr Dermatol 2017;34:2:209-210.
20. Ali MJ. Pediatric acute dacryocystitis. Ophthal Plast Reconstr Surg 2015;31:5:341-7.
21. Tang D, Lobo BC, D’Anza B, Woodard TD, Sindwani R. Advances in microdebrider technology: Improving functionality and expanding utility. Otolaryngol Clin North Am 2017;50:3:589-598.
22. Setliff RC, Parsons DS. The “Hummer”: New instrumentation for functional endoscopic sinus surgery. Am J Rhinology 1994;8:6:275-8.
23. Dogan E, Yüksel NG, Ecevit MC, et al. Microdebrider assisted endoscopic marsupialization of congenital intranasal nasolacrimal duct cysts. Int J Pediatr Otorhinolaryngol 2012;76:4:488-91.



