Management of thyroid eye disease has shifted dramatically from conservative measures—observation and surgery—to targeted biologic therapy with a focus on cosmesis and quality of life. Surgical innovations have also allowed for more profound results with significantly less risk of complications. Treatments for active-phase disease have also multiplied, which significantly affects quality of life for these afflicted patients.
Last month, in part one of this two-part series on TED, I discussed the keys to diagnosing TED. Here, in part two, I’ll discuss the best techniques for managing patients with TED.
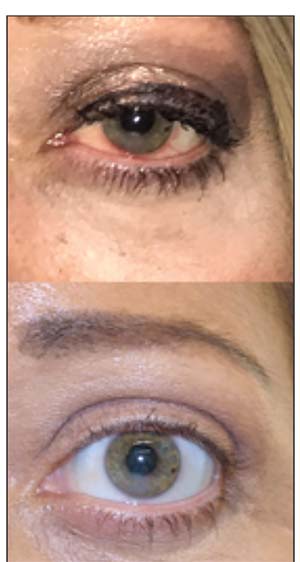 |
| Figure. 1. Eyelid swelling and ocular redness in a patient with thyroid eye disease (top). Improvement in redness and swelling after orbital steroid injection (bottom). |
Medical Therapies
Medical therapy can be a good initial, noninvasive way to manage certain patients with TED. Following are the pros and cons of the various medical approaches.
• Vitamins. As discussed in part one of this series, data has shown that supplementation of selenium has reduced the severity and progression of disease in patients with mild thyroid eye disease.1 Dosing is at 100 µg daily, and is best started as early as possible in the course of the disease, preferably within six months of onset. While there have been no studies of vitamin D supplementation in patients with TED, laboratory studies have shown an anti-inflammatory effect. Checking vitamin D blood levels and supplementing appropriately does little harm to TED patients, and may even be helpful. Beyond this, a good multivitamin and a healthy diet low in fats and processed food is beneficial for both gastrointestinal and overall health. And of course, the number-one piece of advice for patients: Stop smoking.
• Topical medicines. Topical eye drops are chiefly prescribed to treat ocular surface disease. In the early, active phase of TED, ocular surface inflammation contributes to dry eye; this often responds to a low-dose topical steroid such as loteprednol or fluorometholone. In the later, stable phase of the disease, chronic exposure is the main cause of dry eye and can be addressed with lubrication—a regimen of artificial tears, gel and nighttime ointment is often necessary. While surgery may eventually be required, an aggressive topical regimen can greatly improve the quality of life for patients suffering with TED.
• Steroids. Steroids are excellent for reducing symptoms in TED, but aren’t disease-modifying; rather, they improve soft-tissue symptoms in the active phase until the body can pass into the stable phase of the disease.
Steroids can be given as direct injections into the orbit, as pills or as intravenous infusions. Direct injections can be given as often as every four weeks in the clinic, and can provide significant symptomatic relief for patients with congestion, eye pain or a dull ache. They are particularly helpful if the disease is asymmetric or if a patient has diabetes or another health condition that makes systemic steroids risky (Figure 1).
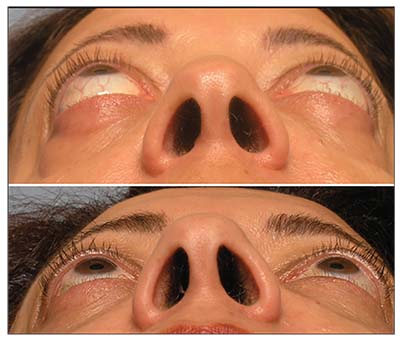 |
| Figure 2. Bilateral proptosis in a patient with thyroid eye disease (top). Dramatic reduction in proptosis seen after orbital decompression surgery (bottom). |
In Europe, nearly every patient with a diagnosis of thyroid eye disease receives intravenous infusions of steroid once weekly for 12 weeks.2 Intravenous steroids have been shown to have a greater efficacy than oral steroids (80 percent vs. 50 percent). While this doesn’t cure the disease, it does reduce the clinical severity and improve the patient’s quality of life. However, 10 percent of patients are resistant to steroids. Steroids also carry the significant risks of liver failure, diabetes, insomnia, psychological changes and even death (if the cumulative dose exceeds 6 to 8 g).
Since the treatment doesn’t alter the course of the disease, the decision of whether or not to undertake a course of intravenous steroids after a new diagnosis of thyroid eye disease is a very personal one and the patient and physician should discuss it in detail. The exception is for patients who are experiencing vision loss: Up to half of patients with optic nerve damage from thyroid eye disease may be able to avoid immediate surgery, or avoid surgery altogether, with a course of intravenous steroids.3
• Orbital radiation. This may sound scary, but it’s a relatively gentle treatment considering the small doses that are used for TED. Radiation is helpful in patients who have persistent inflammation in active disease, and it’s synergistic with steroids (i.e., the combined effect of radiation and steroids together is greater than either alone). Treatment is typically given in 10 sessions over two weeks, with a total dose of 20 Gy, which is well below the dose required to cause retinal damage (35 Gy) or nerve damage (50 Gy). Common side effects can include corneal dryness or development of an early cataract. Radiation may be particularly helpful in patients who have strabismus and double vision,4 although it doesn’t cure or shorten the course of active thyroid eye disease.
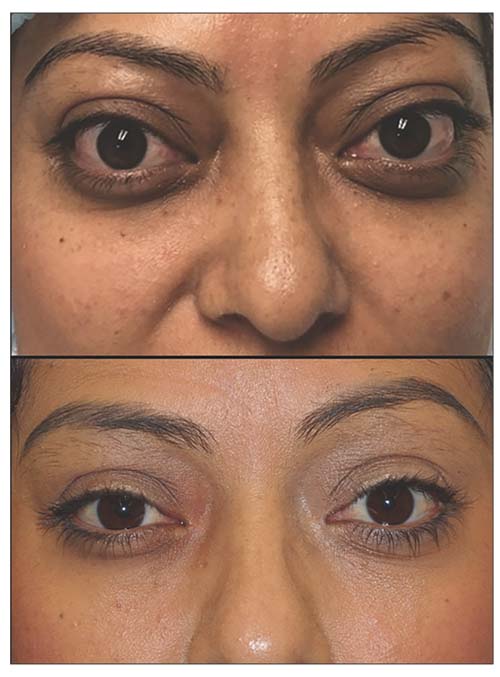 |
| Figure 3. Patient with visual compromise, orbital congestion and pain from thyroid eye disease (top). Improvement in cosmetic appearance, as well as vision, orbital congestion and pain after decompression surgery (bottom). |
• Biologic therapy. Biologic therapy, or customized molecular medicines that specifically target the abnormal biochemical pathways in thyroid eye disease, are a promising hope for the future. These include currently available medicines such as adalimumab (Humira), infliximab (Remicade) and etanercept (Enbrel), as well as many medicines yet to be approved. Unfortunately, these drugs are quite expensive, precluding a large-scale randomized trial from being conducted. At the same time, insurance companies are hard-pressed to approve a medicine that hasn’t been proven in a trial. As such, physicians are left with weak studies that are little more than anecdotes about clinical improvement with these medicines.5
Rituximab is a medicine well-described in its use against lymphoma. It targets the immune cells that produce antibodies (B-cells), and physicians were initially very hopeful that there would be a clinical effect in patients with TED. Two large, randomized, controlled, concurrent trials were conducted: one in Europe and one in the United States.6,7 Unfortunately, the results were conflicting, with the European study suggesting a beneficial effect of rituximab6 and the United States study showing no improvement.7 While there are technical explanations for these confusing results, the bottom line remains that there is no clear evidence that rituximab improves the course of TED.
Tocilizumab (Actemra, Genentech) is a potent biologic medicine that was initially used for other severe autoimmune diseases such as giant cell arteritis. There have been case reports of improvement in TED,8,9 and Genentech recently completed a randomized, controlled trial, the results of which are pending.
The most exciting drug to be developed in recent memory for thyroid eye disease is surely teprotumumab (RV 001). Teprotumumab is an antibody directed against IGF-1, the growth factor pathway associated with the thyroid-hormone receptor. Teprotumumab is the only medicine to date proven to reduce overall clinical severity and proptosis, and provide a sustained response.10 In other words, teprotumumab can halt progression of active disease and reverse any changes associated with TED, and the effects are long-lasting. Now that this trial has been published, the medicine is in the hands of the Food and Drug Administration, where the approval process could take several months or even years. The drug is still available to patients on an out-of-pocket basis if necessary, however, and the manufacturer can provide discounts based on need and disease severity.
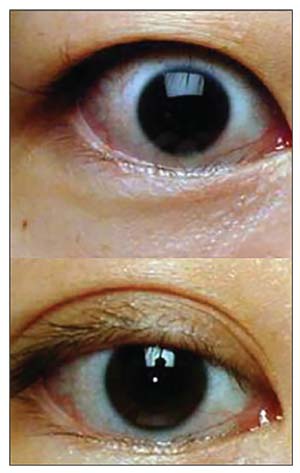 |
| Figure 4. Eyelid retraction (top) with improvement in upper lid position after eyelid retraction surgery (bottom). |
Overall, biologic medicines and targeted molecular therapy are the wave of the future. More research is needed, but patients with TED live in exciting times, as these new medicines can truly be life changing.
Surgical Therapies
Surgery for TED is often necessary, but is usually delayed until the disease is in its stable phase. There’s a small (<10 percent) risk of reactivating the active phase of the disease after surgery, though this is rare. The surgical options consist of the following:
• Orbital decompression. Orbital decompression surgery is the mainstay of rehabilitation for TED; it can improve nearly every aspect of the disease, from vision-threatening optic nerve damage or corneal exposure (Figure 2) to cosmesis (Figure 3). Quality of life is often better after surgery, since orbital congestion, pain and dry eye can improve. Common complications include double vision and scarring (5 to 25 percent, depending on the technique used), while rare complications include vision loss (<0.5 percent, partial or total in the operated eye), though these can be minimized with newer, minimally invasive techniques.11,12 Surgery is performed under general anesthesia through invisible skin incisions hidden in the natural folds of the eyelids, takes 60 to 90 minutes, and is performed on an outpatient basis.
• Strabismus surgery. Surgery to adjust the extraocular muscles and improve double vision is commonly performed. However, this can be much more complicated than typical strabismus surgery, and needs to be performed by a surgeon who is experienced in thyroid eye disease. This surgery takes between 30 to 60 minutes, can be performed under general or twilight anesthesia, and is done on an outpatient basis. Patients can have significant improvement if the muscles aren’t too scarred.
• Eyelid surgery. Surgery to improve eyelid retraction is often the final step in rehabilitation. This step can also be the most temperamental, as the eyelid structures are incredibly minute and unpredictable. However, significant improvement can be achieved (Figure 4). Surgery is performed under twilight anesthesia, can take between 30 to 60 minutes, and is done on an outpatient basis.
• Cosmetic surgery. While thyroid eye disease primarily affects the tissues inside the orbit, there are significant changes in the skin and fat in the eyebrows, cheeks, neck and other areas of the face. In fact, the eyebrow and cheek fat tends to grow alongside the orbital fat, creating a characteristic “hourglass” appearance (Figure 5). These various changes can be addressed with a combination of lasers, fillers, botulinum toxin (i.e., Botox), or even surgery for the eyelid, eyebrow, face and neck. Great care must be taken when undergoing cosmetic surgery in the context of thyroid eye disease: Treating a TED patient like any other cosmetic surgery patient can, at best, lead to a hollow, unnatural look and, at worst, lead to severe corneal exposure and loss of vision or even loss of the eye.
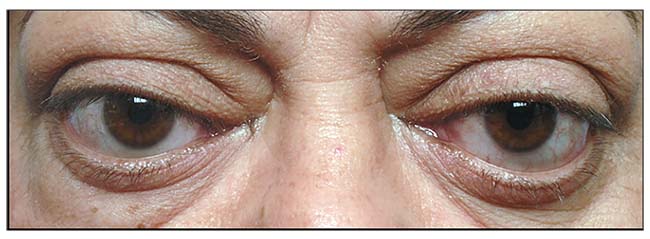 |
| Figure 5. Fat growth in the brows and lower lids and cheeks after thyroid eye disease, causing an “hourglass” deformity. |
Future Directions
With all the exciting research and medical and surgical innovations that have come to fruition in the past several years, it’s an exciting time for patients with thyroid eye disease. In an ideal world, we’d be able to predict exactly which patients with Graves’ disease will develop thyroid eye disease, treat them with a medicine to prevent TED, and reverse any clinical manifestations that may have already occurred. This goal is within reach, since game-changing new medicines that can halt and reverse symptoms of TED are in the pipeline, and the focus has shifted from simply preserving vision to enhancing such things as cosmesis and quality of life. REVIEW
Dr. Ramesh is a clinical assistant professor of ophthalmology at Sidney Kimmel Medical College at Thomas Jefferson University, and a member of the Wills Eye Hospital Orbital and Oculoplastic Surgery Service. He practices at Eye and Facial Plastic Surgery Consultants in Langhorne, Pennsylvania. His areas of interest include TED, facial trauma and cosmetic facial and eyelid procedures.
1. Marcocci C, Kahaly GJ, Krassas GE, et al. Selenium and the course of mild Graves’ orbitopathy. N Engl J Med 2011;364:20:1920-31.
2. Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy guidelines for the management of Graves’ orbitopathy. Eur Thyroid J 2016;5:1:9-26.
3. Wakelkamp IM, Tan H, Saeed P, et al. Orbital irradiation for Graves’ ophthalmopathy: Is it safe? A long-term follow-up study. Ophthalmology 2004;111:8:1557-62.
4. Prummel MF, Terwee CB, Gerding MN, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab 2004;89:1:15-20.
5. Ayabe R, Rootman DB, Hwang CJ, et al. Adalimumab as steroid-sparing treatment of inflammatory-stage thyroid eye disease. Ophthalmic Plast Reconstr Surg 2014;30:5:415-9.
6. Salvi M, Vannucchi G, Curro N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: A randomized controlled study. J Clin Endocrinol Metab 2015;100:2:422-31.
7. Stan MN, Garrity JA, Carranza Leon BG, et al. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 2015;100:2:432-41.
8. Perez-Moreiras JV, Alvarez-Lopez A, Gomez EC. Treatment of active corticosteroid-resistant graves’ orbitopathy. Ophthalmic Plast Reconstr Surg 2014;30:2:162-7.
9. Sy A, Eliasieh K, Silkiss RZ. Clinical response to tocilizumab in severe thyroid eye disease. Ophthalmic Plast Reconstr Surg 2017;33:3:e55-e7.
10. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017;376:18:1748-61.
11. Ramesh S, Nobori A, Wang Y, et al. Orbital expansion in cranial vault after minimally invasive extradural transorbital decompression for thyroid orbitopathy. Ophthalmic Plast Reconstr Surg 2018; 2018 Jun 6. (epub ahead of print).
12. Ramesh S, Eichhorn K, Leibowitz S, Goldberg R. Bony regrowth after deep lateral orbital decompression. Ophthalmic Plast Reconstr Surg 2018;34:6:533-35..



