Optical coherence tomography has become a powerful tool for analyzing the retina and other ocular structures. Now, it’s proving its usefulness as a tool for evaluating the anterior segment as well. Areas in which anterior segment OCT is showing promise include evaluation of the angle in glaucoma patients; evaluation of the cornea in refractive surgery patients—particularly LASIK patients; measuring the anterior chamber for certain phakic intraocular lenses; and as an adjunct in other operations involving the cornea, such as PTK and corneal transplants. Here, three experts share their advice on making the most of this technology in the clinic, and offer a glimpse into other uses for anterior chamber OCT that are being developed.
AS-OCT and Glaucoma
Anterior segment OCT is rapidly becoming a valuable tool for managing some glaucoma patients. Sunita Radhakrishnan, MD, research director of the Glaucoma Research and Education Group at the Glaucoma Center of San Francisco, and Joel S. Schuman, MD, Eye and Ear Foundation Professor and chairman of the department of ophthalmology at the University of Pittsburgh School of Medicine, and co-inventor of OCT, note that this technology can be useful in multiple ways:
• Evaluating narrow angles. Drs. Schuman and Radhakrishnan both say they primarily use anterior segment OCT to assess angle structure in glaucoma patients with narrow or suspicious angles—angles that are not completely occludable, but not wide open either. “In this situation I use the Visante, the one I have the most experience with,” says Dr.
Radhakrishnan. “It’s a very useful tool, because it gives you cross-sectional views of the angle without any contact with the eye. Many times I see people who were referred for open-angle glaucoma who actually have angle-closure glaucoma.”
Dr. Schuman notes that in addition to the Visante, a number of spectral domain OCT devices currently avail-
able in the United States, including the Cirrus and RTVue, can be used to image the anterior segment. “They provide a nice image of the cornea,” he says, “but the wavelength is shorter than the Visante’s, which is approximately 1,310 µm; the others are about 840 µm. Because of the shorter wavelength, the penetration is not as good. That’s most evident when you’re looking at angle structure; often, you can’t see into the recesses of the angle with the spectral domain devices.”
Both doctors note that anterior segment OCT’s ability to perform scans in the dark is a significant help with suspicious angle patients. “That means scanning with the lights on, turning the lights off, waiting a couple of minutes and scanning again,” says Dr. Schuman. “An angle that is occludable will be narrow or closed in the dark. And unlike the classic darkroom test in which you put a patient facedown in the dark for an hour and measure his pressure before and after, this test just takes a couple of minutes. Of course, it only provides a structural assessment, not a functional assessment—it doesn’t tell you anything about the consequences of an angle narrowing, such as how much the IOP rises as a result. But it gives us enough information to know whether or not a laser iridectomy is indicated. And it lets us show the patient that his angle does close in a dark environment.”
Dr. Radhakrishnan agrees. “One of the big advantages of anterior segment OCT is that it allows us to perform imaging in the dark,” she says. “With gonioscopy, you have to use some slit-lamp illumination, which is a potential source of error. My protocol is to image four quadrants in both light and dark conditions. When reviewing the scans, I look at the overall anterior segment anatomy, the iris configuration and whether iridotrabecular apposition is present or not.”
Dr. Radhakrishnan adds that she seldom uses ultrasonic biomicroscopy as the first imaging method in patients with occludable angles. “UBM is more cumbersome to perform,” she says. “It’s a contact procedure; the patient has to lie down; the resolution is lower; and limbus-to-limbus scanning cannot be done. There are newer UBM devices that overcome some of these limitations, but I have limited experience with those.”
Dr. Radhakrishnan also notes that anterior segment OCT can tell the doctor a little bit about the mechanism in angle closure. “With a pupillary block, you’ll see a convex iris configuration,” she explains. “The iris bows forward. In plateau iris, you see a flat iris plane. Unfortunately, anterior segment OCT doesn’t show the ciliary body.”
Dr. Schuman advises caution when considering the possible mechanism, however. “You can get a sense that the problem is plateau iris from the shape of the iris, the cross-section that you see on anterior segment OCT,” he says. “But you won’t see the ciliary processes pushing the iris up as you would with ultrasound biomicroscopy. Nevertheless, anterior segment OCT does have the advantage of being noncontact. I also think—and this is anecdotal—that it provides a more physiologic assessment of the angle structure.”
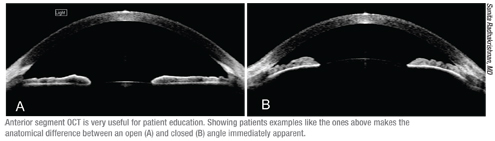 |
Dr. Radhakrishnan agrees. “When discussing laser peripheral iridotomy, I bring these patients to the room where we perform Visante scanning,” she says. “We sit in front of the screen and I show them how their angle looks. I also show them an example of an open angle, which helps them to understand the difference between an open angle and an occludable angle. I explain why we do an iridotomy and how it works. Patients have a much
better understanding when they’re looking at scans of their own eye rather than at an eye model or a diagram.”
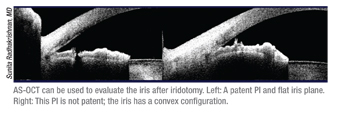
• Evaluating the iris after iridotomy. “In clinical practice, most patients have multiple mechanisms for angle closure; the first step is usually laser iridotomy to relieve pupillary block,” says Dr. Radhakrishnan. “After an iridotomy, I’ll sometimes use OCT to make sure the iridotomy is open. If an iridotomy is small, it may transilluminate at the slit lamp but not actually be full-thickness. With the Visante you can see whether or not it goes all the way through. [See example, above.] Another clue about the adequacy of the iridotomy is the iris plane; this should flatten with elimination of pupillary block.
“I also repeat gonioscopy after iridotomy, to see how much the angle has opened,” she continues. “Studies have shown that about 25 percent of angles still look narrow after an iridotomy. With the Visante you can look for signs of plateau iris or a high crystalline lens rise. I’ll perform UBM imaging in cases with persistent angle closure after iridotomy or cases in which I suspect an irido-ciliary mechanism such as cysts or plateau iris.”
• Pachymetry. “You can get corneal thickness measurements using anterior segment OCT,” notes Dr. Radhakrishnan. “The Visante, for example, can produce a pachymetry map. Our standard approach is ultrasound pachymetry, but in some abnormal corneas, when we’re unable to get measurements with ultrasound pachymetry, I’ve used the Visante. Keep in mind, however, that the OCT numbers do not always agree with the ultrasound readings.”
Pearls: AS-OCT and Glaucoma
 |
• Use OCT as a complement to gonioscopy, not a replacement. “As a glaucoma specialist, I would not say that the Visante replaces gonioscopy,” says Dr. Radhakrishnan. “You get a lot of information from gonioscopy that you don’t get from the Visante. You can indent the eye and see how much the angle opens; you can assess trabecular meshwork pigmentation; you can see abnormalities like neovascularization. These are all things you can’t do with the Visante.
“The Visante gives you a cross-sectional view—a different view from gonioscopy,” she continues. “But by itself, that’s not enough. With the Visante I only look at four cuts out of a 360-degree circle, and I want to see a 360-degree view. It’s a practical impossibility to do every degree with the Visante. Furthermore, OCT usually shows more angle closure than gonioscopy, so treatment decisions can’t be based on imaging alone. Gonioscopy is critical when deciding whether or not an iridotomy should be performed.
“Gonioscopy is my main resource,” she concludes. “I consider Visante to be an adjunct. If an angle looks occludable to me by gonioscopy, I do the Visante as a confirmation. I want to see whether there is apposition, especially in the dark.”
Dr. Schuman agrees that OCT is most useful for glaucoma in conjunction with other tools; for example, he performs gonioscopy on all of his glaucoma patients. “In my opinion, all patients deserve to have gonioscopy done at least once in their lives,” he says. “It takes a few minutes to do, but there are things you can see with gonioscopy that you can’t see with anterior segment OCT, such as color, pigment and blood vessels. Gonioscopy allows me to assess not only the visual appearance of the angle, but also the status of the trabecular meshwork.
“At the same time, it’s a subjective assessment,” he notes. “That’s a big advantage of anterior segment OCT: It gives you a more objective measure of the structure of the front of the eye. So if I have a suspicion of a narrow or occludable angle, then I’ll do anterior segment OCT and a light/dark test to evaluate the structural changes that occur in a dark environment. If I’m suspicious that there’s plateau iris, or a cyst or tumor pushing from behind causing the narrow angle or angle closure, then I’ll get a UBM.”
 |
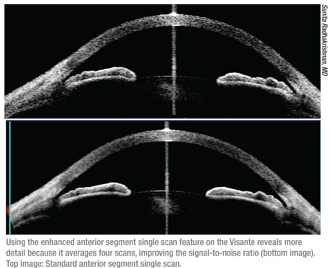
• Check the patient’s head position. “Patients are positioned for scan-
ning with their chin on the chinrest and their forehead up against the bar,” says Dr. Radhakrishnan. “When you make adjustments to the scanning beam, the chinrest moves automatically. If the patient doesn’t move along with the chinrest, you may not see the structure you wish to scan. So if you don’t see any change in the scan window despite your adjustments, the reason is usually an issue of head position. You should always check that.”
 |
• Keep the image horizontal. “Usually, if the patient is looking straight ahead, the image will look tilted on the screen,” says Dr. Radhakrishnan. “If you adjust the fixation a little bit to the side, that will make the image more horizontal. In many cases, you get better visibility on both sides of the angle with a straight image rather than a tilted one.”
• Look for the reflex saturation beam. “If the scan beam is perpendicular to the eye, you’ll see a bright line in the center of the image,” says Dr. Radhakrishnan. “That’s called a reflex saturation beam. An ideal image will be horizontal, with no blink or lid artifacts, and the bright reflex saturation line going through the middle of the scan.”
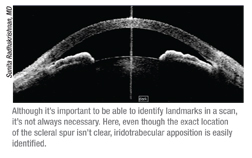
• Don’t discard the scan if the scleral spur isn’t visible. “There are several studies showing that the scleral spur is not visible in 25 or 30 percent of angles scanned with the Visante,” says Dr. Radhakrishnan. “That’s considered a downside. But even when the scleral spur is not clearly visible, you can often make a qualitative judgment about whether or not there’s apposition, based on the rest of the anatomy.”
• Don’t depend too much on the quantitative tools. Dr. Radhakrishnan notes that the Visante has tools for making quantitative measurements of the angle, but says she doesn’t use them routinely. “There’s variability depending on where you place the tool, and there’s no easy way to get to the same position every time,” she points out. “So, when the angle width or area measures smaller six months later, I might just be measuring in a slightly different location.”
Dr. Schuman concurs. “There are various mathematical formulas to determine the actual space between the iris and the trabecular meshwork and the shape of that space,” he says. “However, I don’t find any of the quantitative tools offered for measuring the angle clinically useful. At the practical level, the important factor is simply whether the angle is open, closed or occludable.”
However, Dr. Radhakrishnan admits that she uses the quantitative tools with a few patients. “These are mostly patients who have had iridoto-my and iridoplasty,” she explains. “In those cases I align the scan along some visible landmark such as the iridotomy site or an iridoplasty scar.”
Should general ophthalmologists consider adding anterior segment OCT to their glaucoma armamentarium? “Anterior segment OCT can be useful if you’re a general ophthalmologist and don’t do gonioscopy too often,” says Dr. Radhakrishnan. “Narrow angles and angle closure are definitely underdiagnosed, and anterior segment OCT gives you a quick cross-sectional view. If anything doesn’t look wide open, you can decide to perform gonioscopy, or if you see apposition and don’t do iridotomies, you can refer the patient to a specialist.“
However, Dr. Schuman believes economic realities might make adding anterior segment OCT too much of a burden. “I’m not sure that most comprehensive ophthalmologists would have enough of a call for anterior segment OCT to make it a useful investment for them,” says Dr. Schuman. “They have other expenses that would show more return on investment.”
AS-OCT and LASIK
Because anterior segment OCT provides a detailed look at the in vivo cornea, it’s proving to be a valuable tool for LASIK surgeons, both before and after surgery. David Huang, MD, PhD, Weeks Professor of Ophthalmic Research at the Casey Eye Institute of Oregon Health & Science University, and a co-inventor of optical coherence tomography, has used OCT for a number of cornea-related purposes. (Dr. Huang has co-authored several books on anterior segment OCT, including Anterior Segment Optical Coherence Tomography and Imaging the Eye from Front to Back with RTVue Fourier-Domain Optical Coherence Tomography.)
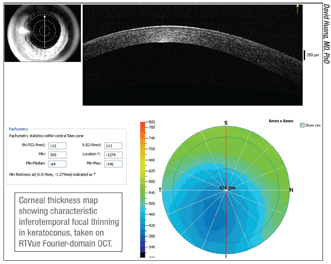 |
Dr. Huang offers two suggestions for increasing accuracy when looking for warning signs of keratoconus.
• Make sure the device is well-centered. “When checking for signs of keratoconus, you’re basically looking for a focal thinning, typically inferotemporally,” says Dr. Huang. “In a normal cornea, the thinnest spot is usually within 0.7 mm of the center. If it’s decentered more than 0.7 mm inferotemporally, that’s a reliable sign of keratoconus.1 The catch is that the decentration can be produced if the operator decenters the scan. So, if you find the thinnest spot in an abnormal position, make sure the scan is well-centered on the pupil. If it is, then I would trust that you’ve detected forme fruste keratoconus. Other metrics such as minimum thickness and minimum minus median can also provide information on focal thinning, and these are not affected by decentration as much—but they may not be as sensitive in very early keratoconus.”
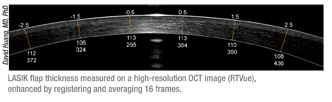 |
A second important use for AS-OCT in LASIK patients is as a way to evaluate the cornea when a LASIK patient needs an enhancement. Dr. Huang says this is especially valuable in a patient who has had a lot of regression. “It’s good to look at the flap thickness and residual stromal thickness to make sure it isn’t a case of ec-
tasia, and to make sure there’s sufficient stroma to ablate,” he explains. “If it’s been many months or even years since the original LASIK, it’s useful to use a Fourier domain OCT, which has higher resolution and will allow you to average a lot of scans.2 This will give you a better chance of detecting a very faint and thin interface that may still exist years later.”
Other Corneal Applications
Dr. Huang uses OCT as part of several other corneal procedures as well:
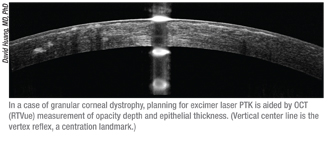 |
• With Intralase and Intacs. “When I’m considering implanting Intacs and using the Intralase to create the channels, I look for patients who have enough thickness, especially in the area in which the inferior Intacs segment would be situated,” says Dr. Huang. “Sometimes if there’s a lot of inferior thinning you wouldn’t want to put in a ring segment. I aim for creating the channel at a depth of 70 percent. The manufacturer recommends putting them in at a depth of 400 µm, but corneal thickness varies, and I don’t want a perforation.”
• For Intralase-enabled keratoplasty. Dr. Huang also uses OCT for IEK when setting the depth of the recipient ablation. “You want to leave a tissue bridge posteriorly, and OCT is good for doing that accurately,” he says. “I usually leave a minimum of 80 µm of tissue, although the amount is just based on personal experience.”
• When measuring for phakic IOLs. Dr. Huang uses OCT to measure the corneal vault and anterior chamber width (from angle recess to angle recess) when implanting a Verisyse phakic IOL. “You can use other methods to make these measurements as well, but OCT may be a little more accurate,” he says. “For this purpose you should use the Visante, which has a wider scan than the RTVue or other converted retinal scanners. Make sure the scan is centered on the corneal vertex. If it is, you’ll see a bright reflection, a vertical bright line.” Dr. Huang adds that this technology should be especially useful with an angle-supported IOL such as Alcon’s Cachet lens, which is currently in clinical trials.
New Uses for AS-OCT
Additional ways to use anterior segment OCT technology are under development:
• Measuring corneal power in post-LASIK cataract patients. “I think this approach works better than using standard keratometry,” says Dr. Huang, who helped to develop the new approach.4,5 “This method bases the power calculation on both the anterior and posterior corneal curvature, the relationship of which has been altered by LASIK. OCT’s high resolution produces very accurate readings, and we’ve had good results using this approach. I think it’s much better than techniques like contact lens over-refraction and the historical method, and in our studies it’s even been a little bit better than newer formulas such as the Haigis formula. Software for this purpose should be coming out in the RTVue system in the near future.”
• Measuring the tear film. Although this work is still mostly showing value in research situations, clinical potential certainly exists. “I’ve done some work using OCT to measure the tear meniscus and tear film,” says Dr. Huang. “Jianhua (Jay) Wang, MD, PhD, at Bascom Palmer, has also done some groundbreaking work in this area. OCT can thus be very useful for measuring the effectiveness of dry-eye therapy in clinical trials.”
• Measuring contact lens fit. Dr. Huang’s group has also done work in this area. “Currently, our group is working with the Boston Foundation for Sight to use OCT for custom design of an ocular surface prosthesis called PROSE, which stands for prosthetic replacement of the ocular surface ecosystem,” he says. “Using OCT we can measure scleral toricity, corneal vault and other parameters that allow a faster and more precise fit, which helps the prosthesis work better and be a lot more comfortable for the wearer.”
1. Li Y, Meisler DM, et al. Keratoconus diagnosis with optical coherence tomography pachymetry mapping. Ophthalmology 2008;115:2159-2166.
2. Li Y, Netto MV, Shekhar R, Krueger RR, Huang D. A longitudinal study of LASIK flap and stromal thickness with high-speed optical coherence tomography. Ophthalmology 2007;114:1124-32.
3. Khurana RN, Li Y, Tang M, Lai MM, Huang D. High-speed optical coherence tomography of corneal opacities. Ophthalmology 2007;114:1278-85.
4. Tang M, Chen A, Li Y, Huang D. Corneal power measurement with Fourier-domain optical coherence tomography. J Cataract Refract Surg 2010;36:12:2115-22.
5. Tang M, Li Y, Huang D. An intraocular lens power calculation formula based on optical coherence tomography: a pilot study. J Refract Surg 2010;26:6:430-7.



