Though small-incision lenticule extraction has been available for several years now, it’s still finding its niche in refractive surgery practices. Due to the way it reshapes the cornea without the use of a large diameter flap, SMILE may be a good choice for very active patients who might be at risk for a flap dislocation, as well as for patients who are at risk for severe dry-eye issues resulting from LASIK’s larger-scale severing of corneal nerves.
Here, I’ll describe the patients with whom I’ve had the most success with SMILE, and the techniques I use to ensure the best outcomes.
Patient Selection
Many of the qualifying factors I use for SMILE come from the LASIK patient-selection process. As a quick review, SMILE is approved for between -1 and -8 D of myopia, with myopic astigmatism up to 3 D. The procedure can only be done with the Zeiss Visumax femtosecond laser. After docking the laser on the patient’s eye, the surgeon uses it to create an intrastromal lenticule by making a posterior and anterior cut. The laser is then used to create a small side cut in the cornea. Using manual instruments, the surgeon dissects the lenticule from the surrounding tissue, then removes it. This removal of the lenticule induces the refractive change.
With the mechanism of the procedure now described, here are the main factors that influence my decision to perform SMILE:
• Ectasia risks. Among the prospective patients who fall into this refractive range, I also consider the corneal thickness. In the United States, we can’t make the overlying SMILE cap thinner than 120 µm-—since it’s 120 µm thick—so as long as the residual stromal bed is thicker than 250 µm, the FDA allows SMILE to be done on that patient. I try not to go below 275 µm of residual stromal bed.
However, I also like to determine the “percent tissue altered,” a LASIK safety metric first introduced by Marcony Santhiago, MD, of Sao Paulo, Brazil, several years ago. PTA is basically the sum of the flap thickness plus ablation depth, divided by the central corneal thickness. Dr. Santhiago has argued that a PTA of 40 percent or greater is a risk factor for the development of ectasia postop. I’m more conservative in my practice, so if a patient has normal topography and tomography, and has less than 38 percent of the tissue altered, I’ll feel comfortable performing SMILE.
• Abnormal topography. Another aspect of the preop screening that comes into play is an abnormality in corneal topography. Though some surgeons might perform SMILE on a patient with abnormal inferior corneal steepening or subclinical asymmetry between the eyes that would rule out LASIK, I wouldn’t. For a patient with borderline or suspect corneal topographies, I’ll tell him that we’re going to monitor him. Most of the time I’ll ultimately perform surface ablation in such cases rather than risk performing a lamellar procedure like SMILE or LASIK. This is important because, based on certain demographics, the incidence of keratoconus may be higher than we expect.1,2 In fact, a literature review of ectasia cases after SMILE that we performed found that most of them had subclinical keratoconus.3 (An apparent increase in the disease’s incidence may also be due to our use of more sensitive instruments and keratoconus indices, such as the Pentacam and the Galilei.)
 |
• Ocular-surface concerns. If I encounter someone with very good corneal topography/tomography who would be a good LASIK candidate, the one thing that might make me shy away from LASIK and lean toward SMILE would be if she had a more noticeable dryness component in her eyes. So, if the patient has inferior staining or some signs of corneal fluorescent staining in the inferior aspect of her cornea, I’ll do my best to bring this under control with our punctal plugs, Restasis/Xiidra or other anti-inflammatory agents over a period of three to six months. However, if she still has signs of dryness even after this therapy, I’ll tend to favor SMILE over LASIK.
This approach is just based on my personal experience. Some studies have shown that SMILE can cause less dryness than LASIK4,5 while at least one study found no appreciable difference between the two in terms of ocular surface disease measurements.6 However, I’ve observed that when I perform LASIK on patients with dry eye, there’s a lot more corneal decompensation postop when compared to SMILE. These LASIK patients with preop dry eye will usually develop a lot more superficial punctate keratopathy in the inferior aspect of the cornea, perhaps due to more corneal nerve dissection.
 |
• The ratio of astigmatism to myopia. If a patient’s refractive error has components of both myopia and astigmatism, and the astigmatic component is 40 percent or more of the refractive error, I prefer to do LASIK in such a patient, if possible. On the other hand, if the proportion of astigmatism is lower, for instance, 1 or 1.5 D, and the myopic component is a higher percentage at -6 or -7 D, I would lean toward SMILE.
This is because I feel our LASIK nomograms are more fine-tuned than our SMILE nomograms. It’s my impression that, so far, even with the nomogram adjustments I have for SMILE, I have to increase the level of myopic correction. For example, if someone is a -7 D myope, I sometimes have to add 7 to 12 percent to the spherical component of the correction. For astigmatism, on the other hand, I don’t have to increase the amount at all; I just go by the measured power of the astigmatism. I’m hoping that, with time, we’ll have more refined nomograms for SMILE, similar in reliability to LASIK’s.
As a subset of this, the overall amount of correction matters too. If you’re just starting, it’s better if you perform it on a patient with a higher level of correction because his lenticule will be thicker and, therefore, easier to work with. Once you’ve done about 50 cases, you can start doing low corrections, such as -1.5 D and less.
 |
Procedure Pointers
Once you’re sure that SMILE is right for a particular patient, there are steps you can take during surgery to make sure you achieve the best outcomes possible.
• Docking the laser/creating the lenticule. Since the Visumax is a femtosecond laser, it needs to be docked using a suction ring in order to perform its photodisruption. The key is to have a picture of the patient’s tomography or topography when you’re sitting at the operating table, so you can see where the angle kappa and fixation point are with respect to the center of the pupil. When you’re docking the laser, the laser is getting closer to the eye, and the patient is looking at the light, you’ll notice that the line of sight might be aligned with the pupil’s center. However, if you have that preop topography/tomography to refer to, it can help you with proper alignment.
I also encourage surgeons to keep the corneal surface moist, but not too moist. If it’s too moist, it will create too large of a meniscus between the applanation glass and the patient’s epithelium. You also don’t want to dock too slowly or too quickly, in order to avoid distorting the corneal surface. Doing this will ensure that you have a good anterior and posterior dissection. When you’re massaging the cap with a moist Weck-Cel sponge, the motion is actually the opposite of LASIK’s: Start at 6 o’clock and brush toward the incision at 12 o’clock.
One challenge with SMILE that we don’t have with LASIK is that the femtosecond laser has no iris registration or ability to compensate for cyclotorsion. When a LASIK patient lies supine, with the excimer laser you can actually rotate the ablation based on the patient’s cyclotorsion or iris registration data. With SMILE, though, since you manually mark the eye before the patient lies down and align the laser for the astigmatism correction, I think there’s enough “noise” in the process that you can’t distinguish 5 degrees of cyclotorsion in your actual attempted correction. So, with SMILE, when you’re doing an astigmatism correction of 2 D on someone with an axis of 93, you really can’t say that you won’t be off by 7 to 10 degrees. Because of this, I feel that SMILE has more efficacy and predictability on lower astigmatic corrections than higher ones.
One of the most common complications surgeons will notice with SMILE is the loss of suction. Depending on when during the photodisruption this occurs, you may have to abort the procedure. If you’ve completed the posterior dissection and lose suction afterward, that’s actually good—you can redock and restart the laser. However, if you lose suction during the posterior dissection, you have to abort. This is because it’s the posterior cut that determines the power of the correction.
If you have to abort, you can convert the patient to a LASIK procedure immediately, creating a flap with the Visumax. Or, you can cancel the procedure, wait one to three months, and then bring the patient back to attempt SMILE again.
• The side incision. One thing to be aware of when you make the side incision is that you can potentially cause an epithelial defect. If this occurs, I advise surgeons to place a bandage contact lens on the surface to allow the epithelium to heal more smoothly at the incision. Doing this decreases the risk of epithelial ingrowth occurring.
• Dissecting the lenticule. Once the side incision is made, you use manual instruments to dissect the anterior aspect of the lenticule, then the posterior, before removing it. Of all the steps of SMILE, this is the one with the steepest learning curve.
First, visualization is important. Though the Visumax has a good microscope, at the moment, the only way to focus it is with a joystick. This poses problems because you also need your hands to perform the dissection. So, as you’re trying to dissect the lenticule, if you lose focus, you have to stop and move your hand over to the joystick to refocus. In most other microscope platforms, however, the focus is controlled by a foot pedal, which allows the surgeon to keep both hands on the eye.
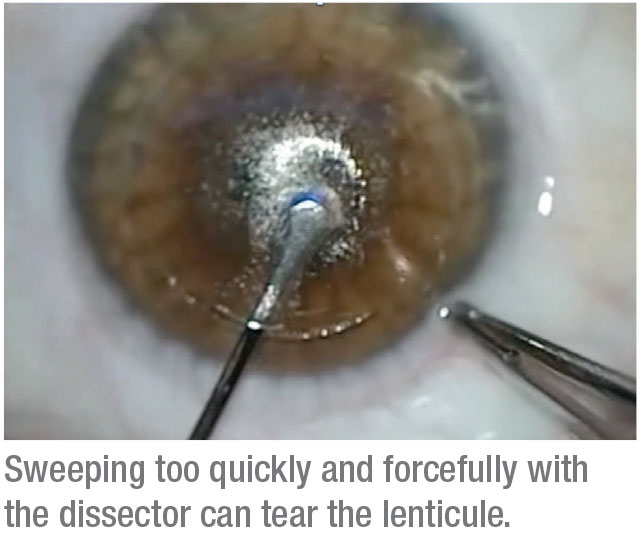
When dissecting, it’s best to start with the anterior plane, then do the posterior. To dissect the anterior plane, use a sharper instrument, something a little longer than a Vanes scissors or Sinskey hook, to find the edge of the lenticule at the incision. The edge is usually inside the incision by a millimeter or so. Once you find the anterior edge of the lenticule with a sharp dissector, switch to a broad dissector and dissect it by a few millimeters with a gentle circular motion, followed by a slow advance of the instrument.
One mistake some surgeons make is that, as soon as they find the anterior plane, they’ll intensely move the instrument back and forth, which can tear the lenticule, especially if the lenticule is for a low-power correction. Instead, gently use the broad dissector to advance the anterior plane toward the visual axis, and then below the visual axis. The movement should be slow enough not to tear the cap or torque the cornea too much. Once the anterior plane is dissected, repeat the steps for the posterior dissection.
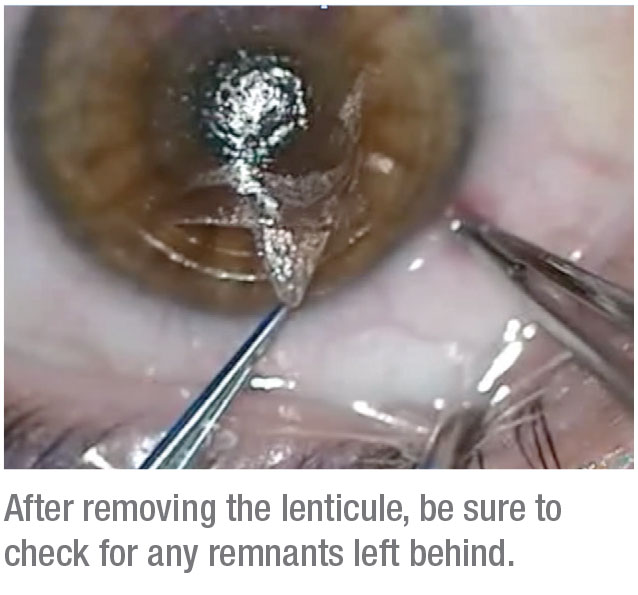
In some cases, it can be difficult to differentiate the anterior and posterior planes, and you may accidentally dissect the posterior first. This isn’t a disaster, but it does make things more challenging. If this happens, it can sometimes be easier to move to a surgical microscope to find the anterior plane and complete your dissection.
If you don’t do a systematic dissection, you can sometimes end up with remnants of the lenticule. These can cause residual refractive error, so it pays to be thorough when dissecting.
• Postop regimen. In addition to a topical antibiotic, it’s important to put these patients on topical steroids immediately, so on the first day, they take a steroid such as fluorometholone every hour. We continue the steroids for four weeks: q4h q.i.d for a week; t.i.d for a week; b.i.d for a week; then q.d. for the last week. This is necessary because we still see more inflammation than after LASIK, or than is seen by international SMILE surgeons who have access to better software.
Since these patients are on steroids for a longer time than LASIK patients, you have to monitor their intraocular pressure. It’s possible for these patients to develop pressure-induced stromal keratopathy, in which interface fluid masks the true IOP. If you suspect PISK, take peripheral IOP measurements to make sure you’re getting an accurate measurement. If it is PISK, discontinue the steroid and prescribe a glaucoma drug.7
Results and Complications
If you’re thinking about performing SMILE, here’s what to expect:
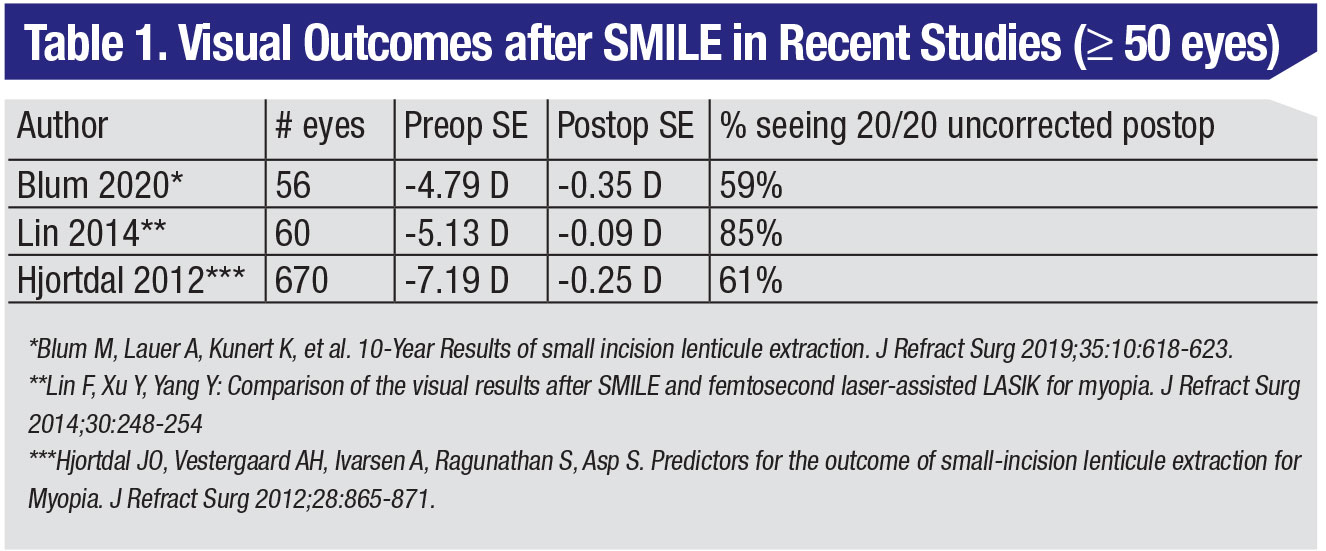
• Results. I’ll speak about my experience first. In the very first set of postop visits—hour one, hour six, 24 hours, and 48 hours—LASIK patients’ vision is better than SMILE by about a line. However, by one month, and then six months, you really can’t see a difference in terms of overall outcomes, efficacy or predictability if your SMILE nomogram is refined and you’re using the most up-to-date energy and spot/line separation settings. Previously, SMILE patients saw about 20/20 or 20/30 in the first four or five days postop. Now, I’d estimate that most SMILE patients are 20/25 or better on the first day. In terms of contrast sensitivity and the scatter index, these measures continue to improve postop until they’re equivalent to a postop LASIK by the third month.
In one prospective, randomized study, 70 patients were randomized to receive SMILE in one eye and LASIK in the other. The average refractive error was -5.3 ±1.8 D in the SMILE eye, and -5.2 ±1.7 D in the LASIK eye. At three months, 99 percent of SMILE eyes and 97 percent of LASIK eyes achieved SE within ±1 D of attempted correction (p=1.0), and the UDVA was 20/20 or better in 84 percent vs. 87 percent of SMILE and LASIK eyes, respectively (p=0.63). At 12 months, the researchers say that SMILE was similar to LASIK in terms of efficacy (85 percent vs. 83 percent UDVA ≥20/20; p=0.81), predictability (99 percent vs. 99 percent within ±1 D of attempted correction SE; p=1.0) and safety.8 At our practice, we compared SMILE to the toric ICL and topography-guided LASIK and found that SMILE may be comparable to toric ICL for patients with high myopia or myopic astigmatism, but SMILE may have a longer visual recovery compared to TG-LASIK than previously indicated.9
• Complications. As mentioned earlier, the most common complication a surgeon will encounter is suction loss during the procedure. There’s also a risk of abrasions at the incision or wound gape due to excessive manipulation. For the former, as noted earlier a bandage contact lens will help prevent epithelial ingrowth. If the wound is gaping, try to approximate the edges of the incision together and then place a bandage contact lens.
A group of surgeons listed their complications after 1,500 SMILE procedures. The most common complications they found were grade 0.5 to 1 haze (7 percent), a dry ocular surface on day one (4.2 percent) and epithelial islands at the incision (0.6 percent). Intraoperatively, there were abrasions at the incision (6 percent), lenticule extraction difficulty (1.8 percent), a minor tear at the incision (1.8 percent) and suction loss (0.7 percent).10
If we see inflammation in the interface, we treat it the same as in LASIK. First, we try to control it with steroids. If that’s not enough, we use systemic steroids. Finally, we wash the interface, though we haven’t had to do that yet.
• Enhancements. Though not necessarily a “complication,” an enhancement isn’t desirable either. Roughly 3 percent of SMILE patients will need an enhancement.11
Currently, the simplest way to perform an enhancement is to do PRK. However, if you want to get creative, the Visumax can convert the SMILE cap to a LASIK flap, and it can create a thinner flap using the existing cap. In the end, though, a PRK is probably best at this point. However, I don’t recommend using mitomycin-C with these enhancements.12
In conclusion, I’ve found SMILE to be a useful addition to my suite of surgical procedures. In the right patients, and using the proper technique, SMILE can provide good outcomes for your patients, as well. REVIEW
Dr. Moshirfar is the director of clinical research at the Hoopes Vision Research Center in Draper, Utah, and adjunct professor at the Moran Eye Center. He is also co-director of the Utah Lions Eye Bank in Murray, Utah. He has no financial interest in any of the products mentioned.
1. Kennedy R, Bourne W, Dyer J. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol 1986;101:267–273.
2. Bamashmus M, Saleh M, Awadalla M. Reasons for not performing keratorefractive surgery in patients seeking refractive surgery in a hospital-based cohort in Yemen. Middle East African Journal of Ophthalmology 2010;17:349–353.
3. Majid M, Albarracin J, Desautels J, et al. Ectasia following small-incision lenticule extraction: A review of the literature. Clin Ophthalmol 2017;15;11:1683-1688.
4. Ganesh S, Brar S, Arra RR. Refractive lenticule extraction small incision lenticule extraction: A new refractive surgery paradigm. Indian J Ophthalmol 2018;66:1:10-19.
5. Wong AHY, Cheung RKY, Kua WN, et al. Dry eyes after SMILE. Asia-Pacific J Ophthalmol 2019;8:5:397-405.
6. Shen Z, Zhu Y, Yao K. Dry eye after small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: A meta-analysis. PLoS One 2016;11:12:e0168081 (online article).
7. Moshirfar M, Somani AN, Vaidyanathan U, et al. Pressure-induced interlamellar stromal keratitis after small-incision lenticule extraction procedure: A case report. Cornea 2020;39:2:254-257.
8. Ang M, Farook M, Htoon H, et al. Randomized clinical trial comparing femtosecond LASIK and small-incision lenticule extraction. Ophthalmology 2020;127:6:724-730.
9. Moshirfar M, Somani A, Motlagh M, et al. Comparison of FDA-reported visual and refractive outcomes of the toric ICL lens, SMILE, and topography-guided LASIK for the correction of myopia and myopic astigmatism. J Refract Surg 2019;35:11:699-706.
10. Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 SMILE procedures. Ophthalmology 2014;121:4:822-8.
11. Liu YC, Rosman M, Mehta JS. Enhancement after small-incision lenticule extraction: Incidence, risk factors, and outcomes. Ophthalmology 2017;124:6:813-821.
12. Moshirfar M, Shah TJ, Masud M. Surgical options for retreatment after small-incision lenticule extraction: Advantages and disadvantages. J Cataract Refract Surg 2018;44:11:1384.



