Easyret Photocoagulator
This 577-nm yellow laser by Quantel (Clermont-Ferrand, France) received FDA clearance in late July 2017. The manufacturer’s proprietary ELBA fiber laser cavity forms the Easyret’s resonator; the laser delivers a 577-nm yellow wavelength in a uniform tophat spot profile to treat ocular diseases such as chronic
 |
| Quantel says that the Easyret is a low-maintenance, ergonomic alternative to solid-state lasers. |
The manufacturer claims that the Easyret’s size and ergonomic setup make it a suitable, durable alternative to solid-state lasers. The Easyret is available with either a Haag Streit- or Zeiss-type slit lamp.
A large touch-screen interface lets users program the appropriate power, pulse, duration, spot size, and treatment beam intensity level for the therapy at hand:
• SingleSpot treatment mode: Traditional focal photocoagulation in one spot, in single, repeat, painting or continuous-delivery modes.
• MultiSpot treatment mode: Multiple consecutive laser spots treated automatically per a preselected customizable treatment pattern (square, macular grid, triple arc, circle or single spot).
• SubLiminal treatment mode: The manufacturer’s proprietary subthreshold micropulse setting divides energy delivery into pulse envelopes with fully customizable on/off durations per laser shot. The company says this affords a high level of control over the thermal effect on targeted tissue.
For more information, visit www.quantel-medical.com.
PASCAL Synthesis TwinStar Laser
In October 2017, Topcon (Livermore, Calif.), announced that the latest addition to its line of PASCAL (PAttern SCAnning Laser) technology, the PASCAL Synthesis TwinStar Ophthalmic
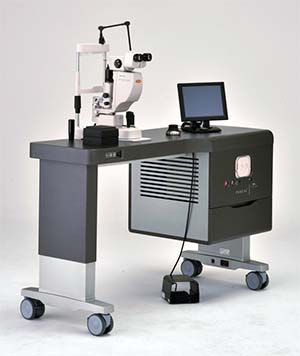 |
| Topcon’s newly-cleared TwinStar laser combines a 638-nm red module with a 577-nm yellow laser module for treatment versatility. |
The newly incorporated red wavelength is intended for targeting the choroid with single-spot treatments. Spot diameters can be set at 50 or 200 µm. The yellow wavelength is intended to perform single-spot photocoagulation in the posterior segment (retina, choroid) as well as pattern-scanning photocoagulation in the non-macular retina. It has settings for single-spot treatment, four spot sizes and a selection of patterns.
The TwinStar also incorporates the Synthesis’ proprietary optional Endpoint Management software for use with the 577-nm yellow module. Endpoint Management allows the user to titrate laser delivery at subthreshold energy levels. This application may help minimize collateral tissue damage in patients who require multiple laser treatments, and may help surgeons and patients feel more comfortable about initiating treatment earlier in the course of disease by helping to limit the potential for cumulative damage.
For more information, visit www.topconmedical.com.
Victus Femtosecond Laser 3.3 Software
Bausch + Lomb (Bridgewater, N.J.) recently got 510(k) clearance from the FDA for a new software package and some hardware modifications for its Victus femtosecond laser that the company hopes will enhance its utility and ease of use in the OR.
The Victus is an Nd:glass solid-state laser in a console that has a computerized interface for the user, and a proprietary Verafit patient interface that keeps the eye still and cornea flat to help the anterior capsulotomy go smoothly and safely. The Victus generates sub-picosend pulses that repeat at 15 kHz. The spot diameter of the beam is less than 6 µm at the target location.
The new 3.3 software platform has a centration feature to guide capsulotomy and lens fragmentation that allows
 |
| In addition to a new 3.3 software package for the Victus femtosecond laser, Bausch + Lomb has updated some ergonomic features to aid workflow. |
For more information, visit www.victuslaser.com.
1. Market Scope press release. Ophthalmic lasers expected to generate $422 million in 2017. July 6 2017. https://market-scope.com/pressrelease/ophthalmic-lasers-expected-to-generate-422-million-in-2017/. Retrieved October 25 2017.



