Workup, Diagnosis and Treatment
Given the onset following trauma, normal orbital CT, and clinical findings, a diagnosis of traumatic CN VI palsy (abducens nerve palsy) was made. Observation with serial examinations for spontaneous recovery was recommended.
Discussion
Horizontal diplopia of acute onset has multiple etiologies, including paresis of the abducens nerve (e.g., ischemia from microvascular disease, ischemia from giant cell arteritis or increased intracranial pressure), dysfunction of the lateral rectus (e.g., laceration or contusion), medial rectus muscle restriction (e.g., medial orbital wall fracture, thyroid eye disease or orbital mass), and neuromuscular junction abnormalities (e.g., myasthenia gravis), among others. In an 80-year-old woman, the two most dangerous etiologies for abducens nerve palsy are giant cell arteritis and increased intracranial pressure. In this case, the patient’s recent motor vehicle accident points to two probable causes: abducens nerve palsy from intracranial injury (with or without increased intracranial pressure) or medial rectus muscle restriction from a fracture of the lamina papyracea. The results of the forced duction and force generation tests ruled out a restrictive strabismus.
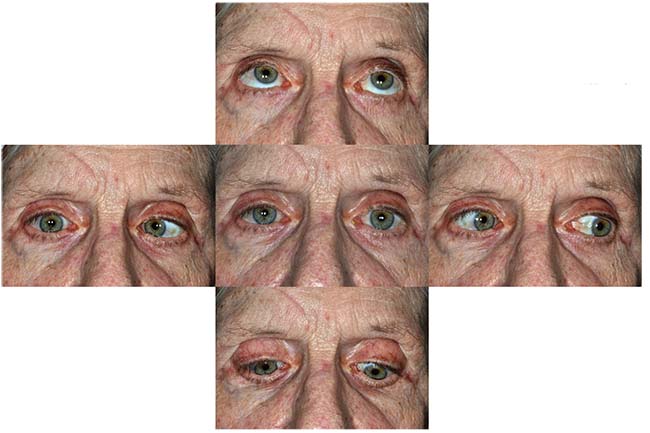 |
| Figure 1. External photograph demonstrating extraocular motility in all fields of gaze. Note the decreased abduction in right gaze. |
Traumatic cranial neuropathies occur in up to 13 percent of all head traumas.1 Furthermore, motor vehicle accidents are the leading cause of intracranial injury that results in cranial neuropathies.1 In the acute setting of diplopia after a trauma, a thorough history detailing the mechanism of trauma and any coexisting injuries should be elicited. Characteristics of the diplopia, including direction—horizontal, vertical, oblique—and binocularity/monocularity should be clarified with the patient. A full ophthalmic examination, including a dilated fundus examination to rule out globe rupture, should be performed. In cases in which the etiology is unclear, forced duction and force generation testing are helpful in differentiating paralytic from restrictive strabismus. Review of head and orbital imaging studies often clarifies the etiology of the diplopia.
Forced duction testing is a relatively simple diagnostic maneuver that checks for restriction (Figure 2A). It doesn’t require patient cooperation and can therefore be performed on patients who are comatose or heavily sedated in the setting of trauma. Viscous lidocaine is highly effective in providing conjunctival anesthesia. Two large-toothed (0.5-mm Castroviejo or Bishop Harmon) forceps are used to grasp the limbal conjunctiva firmly 90 degrees from the muscle of interest. It should be noted that grasping the muscle insertion is painful and runs the risk of a corneal abrasion if the forceps slip. The globe is then moved away from the nidus of suspected restriction. The globe will move smoothly away from a normal extraocular muscle, while an entrapped or enlarged muscle will be perceived by the examiner as resistance to movement.1
Force generation testing checks for extraocular muscle weakness, either from direct muscle injury or from cranial nerve paresis (Figure 2B). However, it requires patient cooperation and cannot be performed in a sedated or comatose patient. The forceps are placed in the same position as forced duction testing; in fact, both tests are typically performed sequentially without regrasping the globe. The patient is asked to look away from the muscle of interest. While grasping the limbal conjunctiva, the patient is asked to look in the direction of action of the muscle of interest. A normally innervated muscle will generate force against the examiner’s grasp, while a paretic muscle will not.1 In this case, the patient’s motility disorder was consistent with right lateral rectus weakness, most likely due to a traumatic abducens nerve palsy. Forced duction testing effectively ruled out muscle entrapment.
The incidence and patterns of motor cranial neuropathies affecting the extraocular muscles following head trauma are important to understand for their prognostic implications. In a 2006 retrospective study, researchers compared 210 consecutive patients who suffered traumatic closed-head injury (CHI). The patients were then subdivided into two groups: those that suffered subsequent ocular motor disturbances and those that didn’t.2 They found that the 95 CHI patients with ocular motor palsies (III, IV or VI) had significantly lower Glasgow Coma Scale scores than those in the control group (GCS mean of 9/15 vs. 13/15 in the control group, p<0.0001), indicating worse overall systemic prognosis. Patients with ocular motor palsies also had a higher incidence of craniofacial fracture and intracranial injury. The study also subdivided the cranial nerve III, IV and VI injuries for comparison. Overall, patients with cranial nerve VI palsies after trauma had the highest average GCS (11/15), necessitated less inpatient rehabilitation, and had fewer intracranial injuries. Patients with cranial nerve III injury after trauma had the lowest average GCS (8/15), with a high incidence of intracranial injury and higher need for inpatient rehabilitation.2
Isolated abducens nerve injury is the most common isolated ocular motor neuropathy following head trauma. The anatomy of cranial nerve VI makes it more susceptible to injury than cranial nerves IV and III.3 Some studies have shown that up to 20 percent of isolated cranial nerve VI palsies are the result of trauma,3 which should not be surprising given the vulnerability of the nerve along the skull base. Cranial nerve VI has the longest course of any cranial nerve, exiting the brainstem at the pontomedullary junction at a sharp angle, travelling along the clivus before making a nearly 90-degree turn into Dorello’s canal, through the cavernous sinus, and finally entering the orbit. Its long course makes it more vulnerable to stretch injury and shearing effects than other cranial nerves.3 Other etiologies of cranial nerve VI palsies to consider in the differential include neoplasm and vasculopathic infarction, which is the most common overall cause (36 percent) of a unilateral abducens palsy.
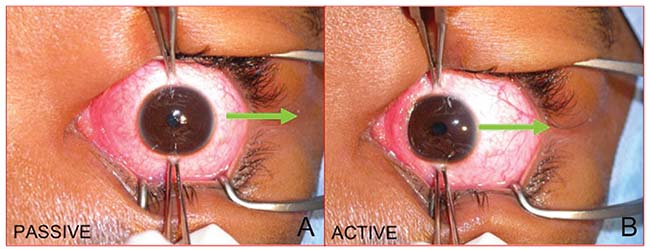 |
| Figure 2. External photograph of a right eye taken from above the patient. A) Forced duction testing used to check for medial rectus restriction. The globe is abducted away from the suspected restriction (arrow). B) Force generation testing to check for lateral rectus paresis. The globe is first adducted away from the suspected paretic muscle. The patient is then asked to abduct the eye and the generated force is graded by the examiner (arrow). Note that this an active test that requires patient participation. |
Unlike unilateral cases, bilateral abducens palsies should raise high suspicion for brainstem lesions and intracranial hemorrhage. Increased intracranial pressure must be ruled out expeditiously in any patient presenting with a posttraumatic abducens nerve palsy; an immediate examination of the optic nerve heads with a direct ophthalmoscope is warranted, and if disc swelling is identified, emergency neuroimaging with CT must be performed.
Management of traumatic abducens nerve palsies is typically conservative, with serial observation over several months. In a study from 1998, the recovery rate of cranial nerve VI palsies after trauma was reported to be 73 percent, with a rate as high as 84 percent in unilateral cases.4 In a 2001 study, researchers identified predictors of nonrecovery, such as bilaterality and a complete palsy at presentation with inability to abduct past the midline; this conferred the highest risk ratio of 9.11.5 In our case, the patient had a unilateral partial paresis, which connotes a relatively good prognosis for recovery. Strabismus surgery should be delayed for at least six months or longer following traumatic palsy to allow for spontaneous recovery. Diplopia symptoms can be managed temporarily with occlusion of one eye or with Fresnel prisms; permanent prisms should be avoided in the acute phase due to their high cost.
In conclusion, diplopia after a motor vehicle accident can be a symptom of many underlying etiologies, the most dangerous of which is increased intracranial pressure. Critical examination techniques include measurement of the strabismus pattern as well as forced duction and force generation testing, which can help differentiate between a paretic and an entrapped extraocular muscle as the source of diplopia. Abducens nerve palsies are the most common traumatic ocular motor nerve palsy. REVIEW
1. Murchison A, Bilyk J, Savino P. Traumatic Cranial Neuropathies. In: Black E, Nesi F, Calvano CJ, Glastone GJ, Levine MR. Smith and Nesi’s Ophthalmic Plastic and Reconstructive Surgery. 3rd Ed. Springer New York. 2012:165-199.
2. Dhaliwal A, West AL, Trobe JD, Musch DC. Third, fourth, and sixth cranial nerve palsies following closed head injury. J Neuroophthalmol. 2006;26:4-10.
3. Elder C, Hainline C, Galetta SL, Blacer LJ, Rucker JC. Isolated abducens nerve palsy: Update on evaluation and diagnosis. Curr Neurol and Neurosci Rep 2016; 16:1-7.
4. Holmes JM, Droste PJ, Beck RW. The natural history of acute traumatic sixth nerve palsy or paresis. J AAPOS 1998;2:265-810.1007/s11910-016-0671-4.
5. Holmes JM, Beck RW, Kip KE, Droste PJ, Leske DA, Pediatric Eye Disease Investigator Group. Predictors of nonrecovery in acute traumatic sixth nerve palsy and paresis. Ophthalmology 2001;108:1457-60.



