It has been estimated that approximately 60 percent of patients who seek treatment are on two or more medications with asymptomatic mild-to-moderate glaucoma. (Ahmed I. MIGS: Unmet needs and opportunity assessment. Presented at: Ophthalmology Innovation Summit, Chicago, April 19, 2012) As such, they do not demand a significant drop in IOP; nor does it seem reasonable to subject them to the short- and long-term risks associated with filtration surgery, including choroidal effusions, shallow anterior chamber, persistent corneal edema, bleb dysesthesia, blebitis and diplopia.1 Therefore, surgical decision-making needs to take into account not only the desired percentage drop in IOP to retard glaucoma progression based upon the amount of optic nerve damage and visual field loss, but also the patient’s quality of life including duration of the postoperative recovery period, follow-up office visits and resumption of daily activities.
Although trabeculectomy is the most commonly performed incisional surgical procedure,3,4 attempts to provide safer alternatives have led to ab externo, blebless non-penetrating surgeries including viscocanalostomy and canaloplasty. This trend was noted in Medicare fee-for-service data from 1996 to 2001, with a 53-percent decline in trabeculectomy in patients without prior surgery, and doubling of trabeculoplasties from 2001 to 2004. Researchers have suggested that trends to lower IOP more aggressively in early glaucoma account for increased use of laser trabeculoplasty.2 They also note that during this period, other than trabeculectomy, other fistulization procedures became very uncommon.
|
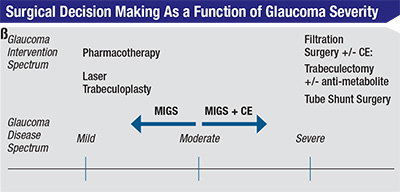
The advent of micro-invasive glaucoma surgery (MIGS), with Food and Drug Administration approval of Trabectome (Neomedix) in 2006 and iStent (Glaukos) in 2012, has created a way to bridge the gap between pharmacotherapy and filtration surgery (See Figure 1). Additional devices are in clinical trials. This group of surgical procedures provides patients with mild-to-moderate OAG an alternative treatment option with a number of favorable characteristics:9
• MIGS represents not only a new trend in angle surgery but a shift from a traditional ab externo surgical approach to an ab interno one with the goal of re-establishing physiologic drainage of aqueous through the conventional outflow pathway via the trabecular meshwork, or enhanced outflow via the uveoscleral pathway or subconjuntival filtration. This approach has several advantages: sparing of the conjunctiva without compromising the option of undergoing a later ab externo incisional surgery should additional IOP control become necessary; surgical precision, given a direct view of the angle structures being manipulated; a stable anterior chamber with minimal impact on the post-refractive state; and ability to combine with cataract surgery via a common corneal incision.
• There is minimal trauma to the eye. Advantages include minimization of inflammation leading to a shortened postoperative recovery period and averting hypotony in the presence of a physiologic distal episcleral venous pressure resistance in the case of Schlemm’s canal procedures.
• The efficacy of the procedure should be sufficient to reduce IOP to the desired target level and/or dependence upon glaucoma drugs. This has the advantage of eliminating the physical burden and associated side effects of administered multiple eye drops.
• MIGS is associated with a high safety profile, eliminating the short- and long-term vision-threatening complications associated with filtration surgery as noted above. This also forms the basis for earlier surgical intervention in the glaucoma disease spectrum.
• Rapid recovery results in minimal disruption in the patient’s quality of life with resumption of daily activities in a relatively short period of time.
New Considerations
Surgeons who wish to embrace this new class of surgeries face two requirements: Not only is there a need to become familiar with angle anatomy in the office setting and for preop planning,11 but also the key to successful angle surgery will be acquisition and mastering of a new skill set—intraoperative direct gonioscopy.10 Surgical microscope and patient head positioning with increased working distance can initially pose a challenge in this setup versus traditional eye surgery performed in a supine position. Some other variables to consider when planning to perform MIGS include: the selection of an appropriate surgical goniolens to ensure good visualization of angle structures; globe stability, particularly with topical anesthesia to counter involuntary eye movements and avoid intraocular complications; and accessibility of surgical instruments via the peripheral cornea.
What is the role of MIGS in surgical glaucoma management? MIGS is a work in progress. Randomized control trials are necessary to determine the efficacy of these angle surgeries in comparison to an established alternative to help in clinical decision-making.4,9,11 Although trabeculecomy has been suggested as the gold standard comparison arm, this may not be appropriate when MIGS is meant to lower IOP modestly in those with mild-to-moderate glaucoma while avoiding complications associated with ab externo filtration surgery.9 The appropriate comparative study arm such as perhaps laser trabeculoplasty, should have comparable IOP-lowering effect and parallel the safety and efficacy of MIGS.
To date, MIGS studies combined with cataract surgery versus cataract surgery alone have shown an overall decreased dependence by one to two medications.13 Two-year data on iStent implantation combined with phacoemulsification versus cataract surgery shows not only a lowering but also maintenance of IOP <21 mmHg on no medications with one iStent vs. control by 53 percent vs. 44 percent, respectively.14 Additionally, the use of multiple iStents (two or three) resulted in a mean IOP < 15 mmHg with a 74 percent decrease in the mean number of drugs (from 2.7 to 0.7) at one year.15
This class of angle surgeries is welcome not only from the patient’s perspective with respect to safety and quicker recovery but also from the surgeon’s standpoint in decreasing the number of planned and unplanned office visits associated with filtration surgery, including those related to complications or requiring surgical revision. In the foreseeable future with MIGS, one can anticipate a controlled, lowering of IOP with one or more of the same or combination of devices working synergistically to lower the IOP to a desired target level in averting progression, while avoiding complications, thereby providing a high benefit-to-risk ratio. With MIGS, glaucoma management is transitioning into becoming a surgically treatable disease enabling intervention at a much earlier stage with an enhanced patient safety profile. REVIEW
Dr. Shareef is an associate professor at the University of Rochester School of Medicine and Dentistry. Dr. Ahmed is an assistant professor at the University of Toronto and clinical assistant professor at the University of Utah.
1. Gedde S, Herndon L, Brandt J, Budenz D, et al. Postoperative Complications in the Tube Versus Trabeculectomy (TVT) Study During Five Years of Follow-Up Am J Ophthalmol 2012;153804-814.
2. Ramulu P, Corcoran K, Corcoran S, Robin A. Utilization of Various Glaucoma Surgeries and Procedures in Medicare Beneficiaries from 1995 to 2004. Ophthalmology 2007;114:2265-2270.
3. Weinreb R, Aung T, Medeiros F. The Pathophysiology and Treatment of Glaucoma–A Review. JAMA 2014; 311:1901-1911.
4. Boland M, Ervin A, Friedman D et al. Comparative Effectiveness of Treatments for Open-Angle Glaucoma: A Systematic Review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:271-279.
5. Inoue K. Managing adverse effects of glaucoma medications. Clinical Ophthalmology 2014; 8:903-913
6. Iordanous Y, Kent J, Hutnik C, et al. Projected Cost Comparison of Trabectome and Endoscopic Cyclophotocoagulation Versus Glaucoma Medication in the Ontario Health Insurance Plan. J Glaucoma 2014;23:e112-e118.
7. Odgerg T, Sandvik L. The medium and long-term efficacy of primary argon laser trabeculoplasty in avoiding topical medication in open angle glaucoma. Acta Ophthalmol Scand 1999;77:176-181
8. Landers J, Martin K, Sarkies N, et al. A Twenty-Year Follow-up Study of Trabeculectomy: Risk Factors and Outcomes. Ophthalmology 2012;119:694-702.
9. Saheb H, Shareef S, Ahmed I. How would we define microinvasive glaucoma surgery? Expert Rev Ophthalmol 2013;8(3):217-219.
10. Shareef S, Alward W, Crandall A, Vold S, Ahmed I. Intra-operative Gonioscopy: A Key to Successful Angle Surgery. Exp Rev Ophthalmol 2014; 9(6):515-527.
11. Francis B, Singh K, Lin S et al. Ophthalmic Technology Assessment: Novel Glaucoma Procedures. A Report by the American Academy of Ophthalmology 2011; 118:1466-1480
12. Au L. Editorial: Are newer surgical interventions for glaucoma making a difference? Br J Ophthalmol 2014;98:1-2.
13. Craven R, Katz J, Wells J, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: Two-year follow-up. J Cataract Refract Surg 2012;38:1339-1345.
14. Belovay G, Naqi A, Chan B et al. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg 2012;38:1911-1917.