Understandably, most surgeons like being able to simply plug numbers into a machine or an online calculator that does the work and produces an answer, without the surgeon having to worry about the details. Nevertheless, the better we understand something, the more likely we are to use it to its best advantage. With that in mind, four experts in this area, all known for their work developing IOL power formulas, share their answers to 10 questions a clinician might ask about these formulas, covering issues such as how they work; how best to use them; and what the future may hold.
1 Is it so bad to just keep using the older formulas?
Most surgeons today are aware that the more recent formulas are capable of producing more accurate power predictions than the older formulas, yet many surgeons continue to use the older ones. To understand how much difference switching to a more advanced formula can make, it’s important to look at outcomes.
Warren E. Hill, MD, medical director of East Valley Ophthalmology in Mesa, Arizona, and creator of the Hill-RBF formula, is in a unique position to compare the outcomes of surgeons using old or new formulas, since he’s reviewed data from more than a quarter-million surgeries (mostly calculated using the older formulas). “After the removal of outliers, and following lens-constant optimization, the average ophthalmologist gets about 78 percent of patients within ±0.5 D of the target refraction,” he says. “Six percent of surgeons are at 84 percent or better. Fewer than 1 percent of surgeons in that database are at 92 percent or better. Different studies around the world have found rates ranging from 55 to 80 percent. So 71 to 80 percent is sort of the acceptable range. That’s what most people achieve using the older formulas.
“Now, as we begin to see the newer biometers and newer formulas, like the Barrett and Hill-RBF, being used by surgeons who also take their time making the measurements and then apply validation criteria, that’s changing,” he continues. “We’re starting to see physician databases in the 90-percent range. Two or three years ago, only 1 or 2 percent of surgeons achieved that; now everybody can potentially reach that level.
“This is a huge sea change in the accuracy of lens-power calculation,” he notes. “We’re all creatures of habit, but fortunately, surgeons are beginning to switch to the newer formulas—what we jokingly refer to as ‘formulas from this century.’ An 80-percent ±0.50 D accuracy level is acceptable, but 90-percent ±0.50 D accuracy is now achievable. We should always strive for what’s achievable.”
Graham D. Barrett, MD, a clinical professor of ophthalmology at the Lions Eye Institute and the University of Western Australia, consultant to the ophthalmology department at Sir Charles Gardiner Hospital, in Perth, Australia, and creator of the Barrett Universal II formula, agrees. “There’s good data to show that the latest generation of formulae, whether it’s mine, the Olsen, Hill-RBF or Holladay II, outperform the earlier generation of formulae,” he says. “Surgeons who are still using the earlier-generation formulae can certainly do better for their patients.”
2 Should I plug my numbers into multiple formulas and compare the results?
This question is one of the few that evokes a range of opinions from the experts. Douglas D. Koch, MD, professor and Allen, Mosbacher, and Law
 |
| Post-refractive-surgery eyes are particularly challenging in terms of predicting ideal IOL power because the alterations to the cornea created by the refractive surgery disrupt the anterior corneal curvature and invalidate correlations that would otherwise help the surgeon estimate posterior corneal curvature and effective lens position. |
Dr. Koch says that’s why he still plugs his numbers into multiple formulas, even in unexceptional eyes. “I’ve been burned by all of the formulas, in terms of errors,” he says. “Postoperative surprises are not the exclusive territory of one formula; I’ve seen it with every single one of them. So in routine eyes I typically run the Holladay I, the Barrett and the Hill. If one of them differs from the others, I tend to lean toward the other two in my IOL selection. Some may think this is no longer necessary, but we keep track of our surgical outcomes: We’re getting 90 percent of our patients within ±0.5 D.”
Dr. Barrett says he doesn’t believe that averaging the predictions of multiple formulas is necessary anymore. “It may be worth looking at two formulas that you have a lot of confidence in, but there’s really no reason to plug your numbers into four or five formulas and try to average them,” he says.
He notes, however, that he sometimes finds it useful to compare the predictions of his own formula and the Hill-RBF formula. “Mine is basically a theoretical, paraxial ray-tracing formula—although there is an element of data-driven enhancement as well, so in that sense it’s a hybrid,” he explains. “Warren’s formula, the Hill-RBF, is purely data-driven, using artificial intelligence. These two formulas tackle the same problem from very different directions. So it’s quite nice to compare those two distinctive methodologies when you look at a particular patient. It’s fascinating how often they come up with a similar prediction, when they couldn’t be more different in how they go about doing the job.”
Dr. Hill agrees that if you’re determined to compare two formulas in a given situation, the Barrett Universal II and Hill-RBF might be good choices. “That’s true for several reasons,” he notes. “First, they’re both current. Second, they’re far more sophisticated than anything that’s come before. Third, they use completely different premises. Dr. Barrett’s formula is based on Gaussian optics; the predictions made by Hill-RBF are derived using artificial intelligence. What’s remarkable is that both of these methods often give recommendations that are within 0.25 D of each other. What this suggests is a convergence of technologies. We’re arriving at something that’s very close to the right answer using completely different approaches.”
Dr. Hill adds an important point regarding the use of multiple formulas for comparison. “If you’re in a situation in which every formula might run into trouble and you want to compare the results, at least make sure the formulas you’re using are well-suited to the task,” he says. “For example, take the extreme axial myope. There are three formulas that do very well with those eyes: the Wang-Koch modification of Holladay I; the Barrett Universal II formula; and the expanded version of Hill-RBF. Comparing the predictions made by those three formulas makes sense because all three of them are uniquely suited to this particular task. You wouldn’t use an uncorrected version of SRK/T with an extreme axial myope—or an extreme hyperope—because it doesn’t do a good job in those situations. If you add formulas that don’t do well into the mix, all you’re doing is adding mathematical noise.”
3 How are the newer formulas different from each other?
With an ever-increasing number of formulas appearing over time, it’s become common practice to categorize them by order of appearance: first-generation; second-generation; and so forth. However, Dr. Koch says he believes this isn’t very useful. “I think we should label formulas in terms of how they calculate the IOL power,” he says. “For example, there are regression formulas, such as the SRK/T. Then there are vergence formulas, based on regular optics. The most commonly used vergence formulas include the two Holladay formulas; the Hoffer Q; the SRK/T; the Haigis; and the Barrett formulas. That group can be further classified by the number of variables they incorporate. The ones that most doctors use—the Holladay I, SRK/T and Hoffer Q—only use two variables. The Haigis uses three variables. Barrett uses five, and the Holladay II uses seven.
“A third category is formulas based on artificial intelligence,” he continues. “Currently, the only formula in that category is the Hill-RBF, which is based on big data. Finally, there are formulas based on ray-tracing. These include the Olsen formula and one from Germany called Okulix, which has not been as well distributed. Other ray-tracing-based formulas exist, but to the best of my knowledge they’re not commercially available.
“The advantage of a ray-tracing formula over a standard vergence formula is that it takes into account the asphericities and other aberrations of the cornea and the lens implant,” he explains. “Vergence formulas use an average value for those factors. As a result, a ray-tracing formula will have some advantages in terms of understanding the power of the IOL and cornea that you’re not going to get with a vergence formula. However, ray-tracing formulas have not achieved their full potential because they still depend on an accurate estimate of effective lens position. Until we do a better job of knowing where the implant is going to sit in the eye, we’re not going to achieve better than 85- or 90-percent accuracy.
“Despite that limitation, the ray-tracing approach is very promising for the future,” he concludes. “That’s what I believe we’ll end up using in the long run. However, at their current level of development those formulas are not outperforming the standard ones, so people don’t have a reason to switch. In fact, a recent article in Ophthalmology showed that the Barrett formula, on average, ends up producing the best outcomes.”1
Dr. Hill explains how his formula, which bases its predictions on the analysis of hundreds of thousands of actual outcomes, differs from the alternatives. “With the theoretical formulas, you enter your numbers and get a power prediction,” he notes. “Then, you implant the lens and hope for the best. My method is different. First of all, it’s not actually a formula; it’s an artificial intelligence algorithm.
“Because it works in this way, it can generate an internal validation process based in multiple pair-wise boundary models,” he continues. “Stated differently, this calculation method is self-validating; you not only get an IOL power, you also get an indication of the calculation’s accuracy. Using boundary models, the Hill-RBF can estimate the likelihood of ending up within ±0.5 D of your target.
“For example, if you go to the online calculator at RBFCalculator.com and put in a sample case, you’ll either get an ‘in bounds’ or ‘out of bounds’ indication,” he explains. “ ‘Out of bounds’ means that, at present, the artificial intelligence database doesn’t have enough data paralleling your specific case to support the calculation at a 90-percent accuracy level. On the other hand, if you get an ‘in bounds’ indication, that means the system has enough data and experience to support a 90-percent ±0.50 D level of accuracy. It’s the first time in ophthalmology that something like this has been offered.” Dr. Hill notes that validating boundary models are frequently used in other fields. “We’re combining ophthalmology with mathematical tools that are commonly used in engineering,” he explains.
Dr. Hill says it took seven years to develop the Hill-RBF method in its present form. “This was a team effort involving 39 investigators in 19 countries, with Doug Koch, MD, and Li Wang, MD, PhD, at Baylor University; Adi Abulafia, MD, in Tel Aviv, Israel; David Goldblum, MD, at the University of Basel in Switzerland; and the engineers and mathematicians at MathWorks as our core investigators,” he says. “The engineers and mathematicians at MathWorks did the heavy lifting for algorithm development.”
4 If I want to switch to a better formula, how do I choose?
Dr. Hill says the first thing to do when choosing a new formula is to listen to people who have experience in this area. “Surgeons shouldn’t make these kinds of decisions in a vacuum,” he says. “Go to meetings. Take CME. Join the ASCRS discussion group—a very good way to stay current in this area. Reading the literature is helpful too, but be careful: Some of what’s been published isn’t grounded in good data. Studies need to have a large, clean database, with standards for refraction and validation criteria applied to the measurements.”
Dr. Barrett points out
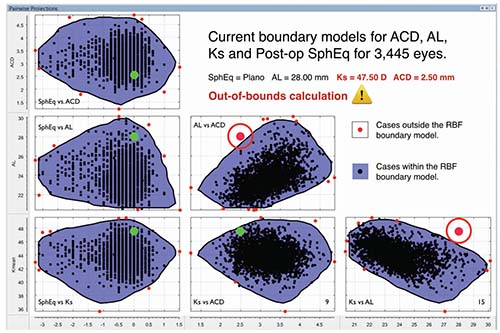 |
| The Hill-RBF method is an artificial intelligence algorithm that bases its predictions on large amounts of previous surgical data. It’s self-validating, using six data pairings to rate the probable accuracy of its lens-power prediction. The label “in bounds” means its database has sufficient data to confirm that its prediction is likely to be accurate; the label “out of bounds” indicates that it cannot make that guarantee. |
Dr. Hill adds that if a surgeon wants to use only one formula, he recommends the Barrett Universal II. “In my opinion, it’s the best theoretical formula currently available,” he says. “I believe it’s the standard.”
Dr. Koch raises an important practical point. “If you’re going to switch to a more advanced formula, the first thing you need to do is make sure you have a device that can take advantage of it,” he says. “The IOLMaster 500 doesn’t measure lens thickness, for example, so you can’t avail yourself of the more sophisticated aspects of the Barrett or Olsen formulas. You can use the Hill-RBF formula, because it doesn’t require lens thickness. But if you want to make the best use of the Barrett and Olsen formulas you need to upgrade to either the Lenstar or the new swept-source devices: the IOLMaster 700, Tomey’s OA-2000 optical biometer, the Argos from Movu, or, in the future, the new Lenstar. All of these devices are very fast and efficient, and they measure lens thickness in addition to the other parameters.
“This practical concern means that moving to a better formula is a two-part process,” he continues. “You also need to move forward with the devices you’re using. Of course, doing so will have other benefits as well: By transitioning from the IOLMaster 500 to one of the newer devices, for example, you’re going to increase the accuracy of your corneal power measurements, particularly with regard to astigmatism. That will help to improve your selection of the appropriate power and result in better alignment of toric IOLs. The newest generation swept-source OCT devices also do a pretty remarkable job of penetrating through dense cataracts. That means you won’t need to rely as much on ultrasound when dealing with a posterior subcapsular or dense cortical cataract. That’s a double plus. Using a more advanced instrument can make a big difference in outcomes.”
5 What’s the best way to deal with very short or long eyes?
Unusual eyes are still problematic when it comes to predicting the best IOL power, and extreme axial lengths are among the more challenging cases.
“In the past it was common practice to use specific formulae for different axial lengths, because some formulae seemed to produce better outcomes in these subgroups,” says Dr. Barrett. “And it’s not just axial length that can challenge formulae; a very flat or steep K can be a source of prediction error if a formula is not really designed to manage those situations. However, most of the latest-generation formulae, which some refer to as fourth- or fifth-generation, perform quite uniformly, even when dealing with short or long eyes or flat or steep Ks. As a result, I don’t believe the practice of using a different formula for different types of eyes is as prevalent today. Most surgeons select their preferred up-to-date formula and are quite happy to use it throughout the full range of axial lengths.”
However, Dr. Barrett admits that even the best formula can run into trouble with very short eyes. “There are reasons for this,” he points out. “For one thing, small errors in measurement have a much greater impact in short eyes. Also, we still don’t know for certain the exact parameters of a given manufactured lens, and small differences have a greater impact in a short eye because the lens has a much higher power than in an average or long eye.”
Dr. Koch agrees. “Short eyes still remain a major source of difficulty,” he says. “These eyes are very susceptible to small variations in effective lens position, and none of our formulas have enough data to do a great job of predicting this. In fact, in my experience, these eyes produce the least accurate outcomes, regardless of which formula you use. We’ve actually found the Holladay I to be just as good in these eyes as any of the so-called advanced formulas.
“The typical error that I see in a short eye is an unexpectedly myopic outcome,” he continues. “The result could be off in either direction, but the big surprises are usually on the myopic side, where the IOL ends up sitting more anteriorly than anticipated. In fact, this outcome occurs primarily—though not predictably—in those eyes that have a shallow anterior chamber preoperatively.
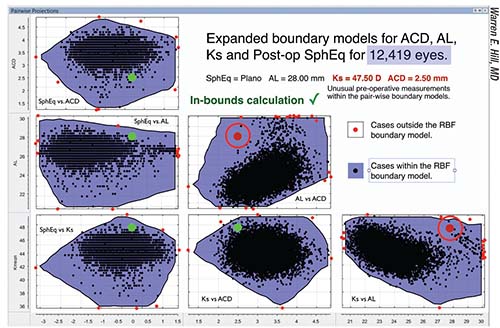 |
| Over time, as the data bank grows larger, cases that would have been “out of bounds” become “in bounds.” For the case shown above, when the database contains 3,445 eyes, two of the six data pairs fall out of bounds (circled red dots). But when more eyes are added to the database (above), all six data pairs now fall within the boundaries covered by the larger database, meaning the new prediction is very likely to be accurate. |
Dr. Koch says that when he’s working with a short eye, he plugs the numbers into five different formulas. “I use the Holladay I, Holladay II, Barrett, Olsen and Hill formulas,” he says. “I look at all of their predictions and select a median value among those five.”
Long eyes can also be challenging. “In terms of long eyes, results have vastly improved with recent advances, beginning with the Wang-Koch formula, and, more recently, Hill and Barrett,” notes Dr. Koch. “Studies have shown mixed outcomes with regard to which of the three is better in this situation. Fram and Masket found the Wang-Koch formula to be more accurate;2 it is a bit more aggressive in that it’s less likely to leave eyes hyperopic, but it’s a bit more likely to produce mild postoperative myopia. It is still our go-to.”
The problem appears to have a lot to do with biometry tending to measure the axial length as longer than it actually is in these eyes. Jack T. Holladay, MD, MSEE, FACS, the developer of the Holladay I, II and Refractive formulas, notes that there’s a tendency to end up with a hyperopic surprise when dealing with eyes deeper than 24 mm. “Factors such as optical biometry using an average index of refraction for the entire eye, or the shape of the IOL, still can’t account for the eye being measured as longer than it actually is,” he says. “So far, no one has been able to explain this error, but whatever the cause is, we can compensate for it by using regressions, fudge factors and adjustments to our formulas.
“Doug Koch and Li Wang were the first to report the axial length measurement problem in long eyes,” he continues. “They published a study presenting two regressions [to help compensate for this], referred to as the ‘1-center’ and ‘2-center’ regressions.3 They recommend using the 1-center regression, which is the more aggressive of the two.
“We recently published an article in Ophthalmology demonstrating that using this regression produces, on average, a myopic error, and confirming that it is rather aggressive,”4 he notes. “I then used the 14,000 cases in the study to generate nonlinear equations for both the Holladay I and II formulas in long eyes. “The results are less aggressive than the 1-center study, producing equal myopic and hyperopic errors. The regression begins at 24 mm—the arithmetic mean of the axial lengths—and has no upper limit. Therefore, in long eyes, we recommend using the Holladay II formula with the Holladay nonlinear regression. This combination can be found, open-access, at hicsoap.com under the Calculator tab. It’s also part of the Holladay IOL Consultant software and is being implemented in the IOLMaster.
“Eventually,” he concludes, “this type of adjustment will evolve to compensate for variations in all of the variables—axial length, K-readings, anterior chamber depth, lens thickness and white-to-white length—to further reduce outcome errors.”
 |
Dr. Hill adds that there’s a lot of “conventional wisdom” that comes with the older formulas regarding which one should be used with a given type of eye—much of which is incorrect. “For example, many surgeons use the Hoffer Q formula for short eyes, when actually Holladay I may do a better job,” he says. “For some reason it got stuck in everybody’s head that you’re supposed to use Hoffer Q. In any case, the newer calculation methods like Barrett and Hill-RBF do much better in those eyes.”
6 What’s the best way to manage post-refractive-surgery eyes?
“The post-refractive-surgery eye remains a disappointing proposition, in the sense that we’re still only getting 70 to 75 percent of these eyes within ±0.5 D,” says Dr. Koch. “I think that will improve in the future as we see more patients who’ve had more uniform ablations, making it a little easier to measure corneal power.”
Why are post-refractive surgery eyes so problematic? Dr. Koch notes three challenges. “The first challenge is knowing what anterior corneal curvature to select, due to variability in this dimension,” he says. “Second, it’s very difficult to accurately measure posterior corneal curvature in these eyes. In a normal eye you can fairly accurately predict the posterior corneal curvature from the anterior curvature, but those assumptions break down after you change the anterior corneal curvature with refractive surgery. That same caveat also applies to using a toric lens in a post-LASIK eye. Finally, the effective lens position calculation is more challenging, since most formulas use corneal power in their equations to estimate ELP.”
Dr. Holladay agrees that the problem is not the IOL formula itself, but the axial length and corneal power measurements that are being used, as well as the estimate of the effective lens position. “As a result, there are three factors that make lens-power prediction in post-refractive-surgery eyes challenging,” he says. “First of all, these are usually long eyes, so the axial length must be adjusted using a regression formula. Second, the cornea has been altered, so standard keratometry is no longer accurate. Third, the current K can’t be used to estimate the effective lens position. Instead, we need to use the K that was measured before the cornea was altered, often referred to as the Double K method.”
Dr. Koch says that if you’re faced with a post-refractive-surgery eye, he recommends getting as many measurements as you can and using formulas found on the ASCRS website. “We like several formulas,” he notes. “We use the Masket formula if we know the change in refraction caused by the previous refractive surgery. We like the Barrett and the Haigis formulas, and we often use the RTVue OCT formula. If you have two or three formulas whose results cluster together, those are more likely to be accurate. We also use intraoperative aberrometry.” He notes, however, that despite having all of these options, he’s seen significant errors with every formula.
“When faced with eyes that have undergone prior refractive surgery, I’d recommend using the online ASCRS calculator that Doug Koch, Li Wang and I created,” says Dr. Hill. “That’s become a very popular tool for this purpose. In addition, surgeons should stay current with the literature. There have been a series of recent articles discussing how to get the best results with these eyes.”
Dr. Barrett notes that there are a multitude of formulas that can be applied with these patients. “Once again, you have to look at the published data,” he says. “Some formulae do better than others with these eyes. What a lot of people do is look at the online ASCRS calculator, because the authors of that—Doug Koch, Li Wang and Warren Hill—critically examine the formulae they include and limit them to the ones that they’ve found provide better outcomes, so you don’t have to choose between 15 or more different formulae.
“Personally, I tend to use my own formula because it can be used for myopic, hyperopic and radial keratotomy eyes,” he continues. “Also, it can be used with or without the history of the change in refraction that was produced by the refractive procedure. If you do know the pre- and post-LASIK, -PRK or -RK refractions, the outcomes will tend to be even more accurate; but if you don’t, the True K formula still gives you a pretty good prediction.”
“The most robust method for estimating the effective corneal power is with topography/tomography where one uses thousands of points on the front and back of the cornea to estimate the ELP,” says Dr. Holladay. “Our group prefers the EKR65 measurement in the Pentacam’s Holladay report. This uses more than 10,000 points on the front and back of the cornea to determine the effective power over a 4.5-mm zone. Results have demonstrated a standard deviation of ±0.56 D for LASIK and ±0.96 D for RK. Sixty-seven percent of the cases will be within this tolerance.”
Dr. Holladay notes that different post-refractive formulas take the corneal refractive error caused by various amounts of treatment and generate a formula to compensate for that error. “The results will depend on the specific laser used for the treatment, since that will affect the postoperative shape of the cornea,” he points out. “Those formulas can work well for a specific laser.”
Dr. Holladay adds that using the K that was measured before the cornea was altered and the refractive change from the procedure, often referred to as the historical method, is still the gold standard for estimating the current corneal power. “If the pre-refractive Ks were accurate and the refractive change is stable—which is normally true for LASIK, though not for RK—then this method works well,” he says. “However, if the refraction has been affected by lenticular changes caused by the cataract, it doesn’t work.”
7 What’s the best way to manage an eye with silicone oil?
Dr. Barrett notes that predicting the correct IOL power in an eye that contains silicone oil is challenging. “The problem with optical biometry in this situation is that the refractive index is different for a lens facing a medium of silicone oil, resulting in inaccurate predictions,” he says. “That makes determining the required lens power quite complex. Using a convex plano lens, in which the back surface of the lens is plano, will help because the silicone oil won’t impact the calculation of required IOL power to the same extent. Unfortunately, those lenses are hard to find.”
Dr. Koch suggests that when you
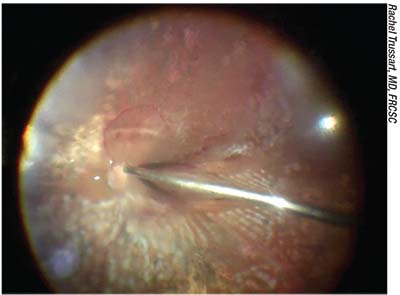 |
| Eyes containing silicone oil are problematic because the oil has a different refractive index that must be accounted for in the power calculation—unless the surgeon is certain that the silicone oil will be removed soon after the cataract surgery. |
“Fortunately, most optical biometers measure eyes with silicone oil with minimal difficulty,” he continues. “If you use ultrasound, then you have to use immersion ultrasound and segment the eye, inserting the refractive index for that particular silicone oil when you’re calculating the axial length. Of course, if you leave the silicone oil in and put in a standard IOL, the patient is going to be very hyperopic until the silicone oil is removed. But being hyperopic for a brief period will be worth it for the patient in the long run.”
“Luckily,” notes Dr. Barrett, “encountering an eye with silicone oil is not a common occurrence, and removing the oil prior to cataract surgery will avoid the complexities of formulae prediction.”
8 Does using a toric lens alter the spherical correction?
“An astigmatism correction [in the IOL] shouldn’t affect the spherical calculation significantly,” says Dr. Barrett. “In theory this could be an issue, because an astigmatism correction could potentially alter the lens structure and the principal planes, impacting the spherical correction. However, the manufacturer can compensate for that. The more sophisticated companies utilize optic designs that ensure that the principal planes of the lens remain equivalent for a given spherical-equivalent lens power, regardless of whether it’s a T2 or T9 toric equivalent. Of course, different companies manufacture their toric lenses differently, so it’s possible that this concern may not always be addressed. But in my experience—using the more common toric lenses—I haven’t found any difference in spherical outcomes using a toric vs. a non-toric lens.”
9 What steps can I take to maximize the likelihood of getting better outcomes?
These strategies will help:
• Optimize your lens constants. Dr. Hill notes that the lens constants provided by the manufacturer can be more than a half-diopter different from a surgeon’s own lens constants. “If you want to improve your outcomes, lens constant optimization is essential,” he says.
• Validate your measurements. Dr. Hill notes that inaccurate measurements are a common source of refractive surprises. “The numbers we generate in the preoperative process come from a biometer, and from time to time the biometer may be wrong,” he says. “There are many places where things can go off the rails, so you have to look carefully at your measurements and make sure they’re correct. The staff should be first in line to check measurement validity, before the patient gets up and leaves the office. Perhaps there’s a serious inconsistency from one measurement to the next, or perhaps there’s something else out of the ordinary. Hopefully the technician picks up on this and either resolves the issue or brings it to the attention of the physician.
“The best way to check the accuracy of your measurements is by using validation criteria,” he notes. “Both Zeiss and Haag-Streit have validation criteria available for the IOLMaster and the LenStar, respectively, so the technician should take the measurements and apply the validation criteria before letting the patient go. The physician should do the same when he or she reviews the measurements, and if something on the final review doesn’t look right, the whole process should stop until the problem is resolved.” (Dr. Hill notes that you can learn more about using the validation criteria by reading his editorial in the July 2017 issue of The Journal of Cataract and Refractive Surgery.5)
• Don’t remove yourself from the process. “Unfortunately, many surgeons let their staff run the show,” says Dr. Hill. “We’re all very busy and typically running behind schedule, so we don’t have time to look over the shoulders of our staff and make sure everything is correct. However, that can lead to a scenario in which everything is automated or delegated. In some practices, a key staff member does all of the measurements and calculations and the physician just selects a lens on a piece of paper.
“That won’t lead to a bad outcome most of the time,” he continues. “However, if there’s something unusual—let’s say the patient has very steep or flat Ks, or an unusual anterior chamber, or a big difference in measurements between eyes—it’s likely to get lost using this kind of protocol. Fortunately, a new form of surgical planning is now emerging, in which the validation is carried out by the planning software in much the same manner as the most knowledgeable and careful surgeons. Zeiss’ new surgical planner Veracity holds the promise of being able to manage this for the surgeon.
“The bottom line is that the physician is the most knowledgeable person on the team,” he continues. “Furthermore, physicians are being judged by their patients and their peers based on their refractive outcomes. The staff needs to look at the measurements and make sure they’re right, but the final arbiter making the final decision regarding IOL calculations needs to be the physician.
“After the surgery, the doctor also oversees the process of tracking outcomes, to ensure that the practice is producing the desired level of results,” he adds. “The surgeon initiates the process of lens-constant optimization, and overall, he or she also offers guidance and keeps the entire process current so the staff doesn’t just keep doing the same thing year after year. The surgeon has to be the person in charge.”
• Spend extra time counseling patients with short eyes or previous refractive surgery. Dr. Koch notes that these patients are most likely to have a refractive surprise. “These patients need to understand that their eyes fall into a category in which it’s difficult to guarantee a perfect outcome,” he says. “One has to be prepared to counsel the patient about the possibility of a two-stage procedure. I always show the patient the calculation sheet to point out that I have many lenses to choose from, and that I cannot be sure which is best. I explain that I’ll use my best judgment about which one to implant, but the outcome could be off. Also, I tell the patient to be ready for the possibility of needing glasses or some form of postoperative modification, and what the cost of that would be.”
• Don’t just keep doing what you’ve always done: Stay informed about the latest developments. “This is a field that changes quickly,” notes Dr. Hill. “What’s considered to be ‘best practice’ shifts at regular intervals. Last month I updated the online Hill-RBF calculator, extending the range of calculation down to -5 D, and the range of in-bounds indications for the very short eye was increased by the addition of 1,000 exceptionally short eyes.
“The point is that you need to stay current,” he says. “Go to meetings; attend webinars; read the journals. That way you’ll know what tools are in the toolbox to help you with the more challenging lens calculations. If you simply do what you’ve always done, inevitably you’ll fall behind the competition. The guy down the street will be keeping up, and you won’t be able to compete.”
10 Will we ever have one formula that works for every eye?
“Running different formulas for unusual axial lengths, Ks, and so forth is time-consuming, and the breakpoints vary with the formulas,” notes Dr. Holladay. “Improving formulas so that they work well for all eyes is our goal.”
Reaching that goal may be a ways off, however—if it’s even possible. “The limitations we have right now are as much about measurement technology as formula shortcomings,” observes Dr. Hill. “There are two problems. First, we have a measurement floor, which means that there’s a limit to how accurate we can be when we’re measuring living tissue. Second, we have a calculation ceiling, which means that we can only be so accurate in the face of technology limitations. As Jack Holladay recently pointed out, we’re starting to see that using current measurement technologies, and given the way intraocular lenses are offered to ophthalmologists, 90 or 92 percent within ±0.5 D of the target outcome is probably the ceiling for accuracy.”
Nevertheless, Dr. Hill says he believes that one day it will be possible to achieve a ±0.50 D accuracy in excess of 95 percent of patients. “This will require an improvement in measurement technology, and calculation methods will have to be optimized to work with these improved measurements,” he says. “In addition, industry may one day be able to deliver us IOLs that have the exact, measured power as part of the labeling process.” (Adjustable lens technology may improve these numbers as well, with lenses such as the recently approved Light-adjustable Lens enabling surgeons to modify the power of the IOL postoperatively.)
Dr. Barrett notes that our ability to measure the posterior corneal surface is evolving. “Being able to measure the eye in a more sophisticated fashion should improve our predictions,” he points out. “We’re on the verge of a new generation of biometers that will do that. New technologies such as Scheimpflug devices and swept-source OCT can measure the posterior cornea quite accurately. That can impact not only our toric predictions but our spherical outcomes as well.
“Now, we’re beginning to have formulae that are customized to utilize this new information,” he continues. “Just in the past few weeks I’ve added an option to my toric calculator, which is online at the APACRS (Asia-Pacific Association of Cataract and Refractive Surgeons) and ASCRS websites, that will allow the user to incorporate that new information. I think that will enhance our outcomes in a variety of segments, not just in toric or post-refractive eyes, but even spherical outcomes in average eyes.”
But will we end up with a single formula that works for every eye? “I doubt it,” says Dr. Barrett. “Of course, most formulae are based on theoretical physics and optics, so you might think they’d all tell us the same thing. The issue, however, is trying to predict where the lens will end up—the effective lens position. That’s often where the author of a particular formula uses a different algorithm; some algorithms are data-driven, some are based on different eye models. To put it another way, the formulae are based on fundamental optics and physics, but there are also elements of hand-crafting, and I don’t think that’s going to change.
“However, there is a convergence,” he adds. “The latest generation of formulae are getting closer to producing precise outcomes. The ceiling that we tend to hit even with a great formula isn’t a sign that the formula is wrong; it’s the result of entering noisy data, including the postoperative refraction (which helps us determine accuracy) and issues such as imperfect lens manufacture. But with good biometry and a good formula, the 90-percent-within-0.5-D goal is becoming achievable.”
Dr. Koch is a consultant for Carl Zeiss, Alcon and Johnson &Johnson Vision. Dr. Hill is a consultant for Zeiss, Haag-Streit, Alcon, Omega Opthalmics, Optos and Veracity Surgical. Dr. Holladay is the developer of the Holladay 1,2 and Refractive Formulas and is president of Holladay Consulting, which is the distributor of the Holladay IOL Consultant Software (hicsoap.com). Dr. Barrett has licensed his formulas to multiple companies, but notes that they are freely available to all online. REVIEW
1. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology 2017 Sep 23. pii: S0161-6420(17)31428-8. doi: 10.1016/j.ophtha.2017.08.027. [Epub ahead of print]
2. Fram NR, Masket S, Wang L. Comparison of intraoperative aberrometry, OCT-based IOL formula, Haigis-l, and Masket formulae for IOL power calculation after laser vision correction. Ophthalmology 2015;122:1096-101.
3. Wang L, Shirayama M, Ma XJ, et al. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J Cataract Refract Surg 2011;37:11:2018–27.
4. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology 2017; Sep 23. pii: S0161-6420(17)31428-8. doi: 10.1016/j.ophtha.2017.08.027. [Epub ahead of print]
5. Hill WE, Abulafia A, Wang L, Koch DD. Pursuing perfection in IOL calculations. II. Measurement foibles: Measurement errors, validation criteria, IOL constants, and lane length. J Cataract Refract Surgery 2017:43;7;869-70.