Inflammation is one of the oldest of medical terms. In fact Celsus, approximately 2,000 years ago, gave us the cardinal signs of inflammation: rubor; tumor; calor; dolor and functio laesa. After two millennia of research (How often do we get to say that?), our understanding of inflammatory mechanisms continues to evolve. Inflammatory processes encompass a broad spectrum of responses designed for protection and preservation of healthy, optimally functioning tissue. Physiological inflammation is intimately linked to innate immunity, the body’s primordial defense system of macrophages and other phagocytic cells that are the specialized first responders to invading pathogens and tissue injury. In contrast, pathological inflammation occurs when regulatory mechanisms fail and the cellular defense system morphs into a suicide machine that attacks and degrades otherwise healthy tissue. The professional cells of innate immunity are often some of the major culprits in pathological inflammation, so it’s clear that in devising therapies for conditions with an underlying inflammatory component it’s critical to strike a balance between suppressing the aberrant actions of immune cells and signals and preserving proper protective functions.
In past columns we’ve discussed chronic inflammation of the type associated with allergy and dry eye, and we’ve also looked at new potential treatments for these conditions. Inflammation is central to so much of what we deal with on a daily basis—keratitis, uveitis, conjunctivitis and dry eye—all of which involve pathological inflammatory responses. This month, we dig a little deeper into some of the fundamental pathways that shape the inflammatory process. In particular, we’ll look at the basic signal transduction events involved in innate immunity, and examine how both professional immune cells and the resident cells of inflamed ocular structures contribute to the overall orchestration of inflammation. Not surprisingly, these efforts will uncover a number of potential targets for therapeutic intervention.
Detecting Pathogen Patterns
The innate immune system is that part of our overall response to foreign invasion or traumatic insult that occurs without provoking an antibody-mediated response. Phagocytic cells such as macrophages, neutrophils or other white blood cells engulf and degrade intruding debris, bacteria and other foreign flotsam. Historically, innate immunity was considered a nonspecific response to any invaders, but research from disparate fields came together in the late 1980s to demonstrate that the immune system used a collection of pattern recognition receptors that orchestrate both innate immune responses and the complex interactions between the innate and adaptive immune systems.1
 |
Recognition of PAMPs or DAMPs leads to activation of PRRs and subsequent triggering of gene transcription factors including NFkB. These direct the production of pro-inflammatory cytokines, chemokines, type-1 interferons, antimicrobial proteins and tissue repair proteins. Pivotal cytokines that initiate and mediate the acute innate response include established therapeutic targets such as TNF alpha, interleukin-1 (IL-1) and IL-6.
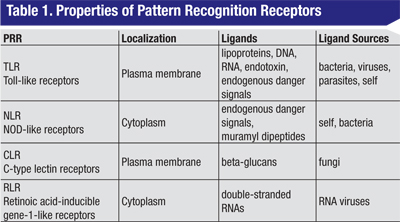
Four different families of PRRs have been identified (See Table 1, above), and each of these receptors acts as a molecular sentry, recognizing molecular patterns displayed by proteins, lipids or nucleic acids derived from potentially harmful sources. The largest and best characterized family of PRRs is the plasma-membrane-associated Toll-like receptors initially discovered in Drosophila.5 To date, 10 human TLRs have been described. A second class of membrane PRRs are the C-type lectin receptors,6 proteins with high affinity for certain complex carbohydrates. The two other families are intracellular proteins: the NOD-like receptors and the retinoic acid gene-like receptors.7 These detect molecular patterns from the products of endosomal processing.
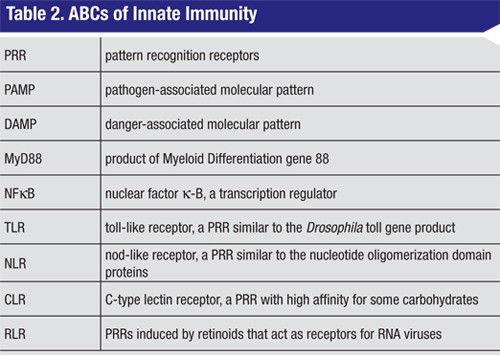
Despite the diversity of the primary signals, the majority of the PRRs act via a small subset of signaling molecules.2,3,8,9 With the exception of TLR3, ligand binding to all of the TLRs leads to recruitment of a common adaptor molecule called MyD88 (See Table 2, p. 75) to the membrane, and subsequent activation of a cascade of intracellular signaling intermediates. These events culminate in transcriptional activation by NFkB or other transcription factors. The net effect is enhanced expression of a variety of target pro-inflammatory genes. TLRs, MyD88 and NFkB therefore represent potential sites for therapeutic intervention in the innate immune response. Of note, TLRs also represent a critical link between innate and adaptive immunity. For example, dendritic cell TLR activation is thought to play a key role in the physiological balance between sensitization and tolerance.10
A Nod’s as Good as a Toll
Another family of PRRs expressed by professional innate immune cells, and also by other cells, is the NOD-like receptors. NLRs are part of cytoplasmic multi-protein complexes called inflammasomes that act as sensors of cellular stress.7,11 Activation of inflammasomes (by stress or by other signals) stimulates a transcriptional pathway similar to that which is activated by TLRs, and includes induction of pro-inflammatory cytokines. Upon activation, changes in NLR conformation lead to recruitment of the enzyme caspase-1 to the inflammasome. Caspase-1 then converts cytosolic pro-IL-1 to the active cytokine, IL-1b. Receptors for IL-1b are widely expressed in immune and non-immune cells and, consequently, IL-1b exerts both autocrine and paracrine pro-inflammatory effects. As with TLRs, IL-1R signals through MyD88 and NFkB to increase pro-inflammatory cytokine levels, including pro-IL-1. Approved biologicals that target IL-1 include the receptor antagonist Anakinra (Kineret, Biovitrum AB) and the soluble IL-1R mimetic Rilonacept (Arcalyst, Regeneron).12
|
Innate Signaling in the Eye
In addition to innate immune cells, non-professional resident tissue cells also actively engage in immune responses through their own repertoire of receptors, signaling molecules and mediators. This resident tissue cell repertoire includes PRRs, activation of which provides mediators that recruit professional innate immune cells to the affected site. Human ocular surface epithelial cells and conjunctival fibroblasts express TLR receptors and respond to activation by TLR ligands.15 Similar to professional innate immune cells, corneal epithelial cells, mast cells and fibroblasts secrete pro-inflammatory cytokines upon activation of TLRs through MyD88 and NFkB signaling. As with dendritic cells, TLR activation of these residents of the conjunctiva participates in local innate responses in addition to more far-reaching, adaptive processes. Beyond the ocular surface, TLRs can be found in a variety of resident tissue cells of the retina, the uvea and in the lacrimal glands.9,15
The endogenous, non-microbial DAMPs are products released from stressed or necrotic cells and damaged tissue. DAMPs include intracellular proteins such as heat shock proteins, extracellular matrix fragments, nucleic acids (DNA, RNA) and purine metabolites including ATP and uric acid.16-18 Recognition of signals of cell stress and death evolved as mechanisms to more effectively fight against pathogens. Tissue injury therefore provides endogenous amplifiers of the inflammatory response. Danger signals may be produced during inflammation caused by infection and by environmental triggers such as pollutants or chemicals, and by mechanical trauma. Reactive oxygen species produced during cell stress are critical danger signals that stimulate the innate immune response. Endogenous danger signal production is a key mechanism by which non-professional tissue resident cells actively participate in the innate immune response.
The innate immune system is implicated in a number of ophthalmic disorders. Uveitis was traditionally considered to be due to loss of immune tolerance to retinal proteins and, therefore, an autoimmune disease. Accordingly, a large body of evidence supports critical roles for Th1 and Th17 cells in mediating pathology in uveitis. However, selective therapeutic targeting of T-lymphocytes has not been as effective a strategy as broadly acting corticosteroids.
The role of innate immunity in uveitis has remained underappreciated despite the fact that it is well known that activation of the innate immune system using microbial adjuvants is required to produce the disease in animals.18 For example, the uveitis seen in Blau syndrome is caused by a specific mutation that activates NLRs.13
Alternatively, activation of innate immunity by prior infection is strongly implicated in Fuchs heterochromic cyclitis. Systemic diseases associated with uveitis in which innate mechanisms are suspected to play critical roles include Behçet’s, Crohn’s and sarcoidosis.
There is also a growing body of evidence that supports a role of inflammation in the pathogenesis of degenerative diseases including Parkinson’s and Alzheimer’s. A similar pattern is seen in diseases of the retina, where evidence demonstrates ongoing inflammation involving innate mechanisms.16 In diabetic retinopathy, one underlying mechanism for tissue damage seems to be a chronic inflammation leading to blockage of retinal capillaries by recruited leukocytes. In animal models, activated circulating bone marrow-derived monocytes have been identified as the primary ischemia-inducing culprit.19 This suggests that innate immune system cells may be part of the trigger for neovascularization and associated visual complications in diabetic patients.
In age-related macular degeneration, the attention of researchers has focused on the role of complement factor gene polymorphisms in individual susceptibility, and the possible associated defects in complement inactivation.20 However, evidence for critical roles by other components of the innate immune system is increasing. Drusen accumulation is associated with increased macrophage and dendritic cell activity21 and the severity of disease is correlated with the presence of macrophages that express a more pro-inflammatory phenotype.22 Cell death, including death of RPE cells, is associated with production of danger signals recognized by membrane-bound and intracellular PRRs, providing a stimulus for inflammatory cytokine production and further macrophage recruitment.
Balancing Immune Signals
Environmental exposure of the ocular surface renders it susceptible to a host of innate immune system triggers. As the first line of defense, the barrier to infiltration imposed by the epithelial cells of the cornea and sclera presents both a physical impediment and a set of physiological sensors that include TLRs. As in other tissues, activation of TLR signaling on the ocular surface initiates a cascade of signaling events that activate innate defenses and set the stage for adaptive immune system intervention.
 |
Links between innate and adaptive immunity can also be found in features of allergic conjunctivitis. Activation of corneal fibroblast and epithelial cell TLRs induces the production of the cytokine thymic stromal lymphoprotein that promotes a pro-allergy, Th2-mediated immune response.26 TSLP promotes Th2 differentiation and proliferation, and enhances other adaptive immune functions of Th2 cells, including activation and recruitment of mast cells and eosinophils.27 In addition, mast cells have been found to express TLRs that, when activated, can synergize with antigen activation of the high-affinity IgE receptor.28
Disruption of homeostasis and compromised ocular surface health in dry eye may be a key determinant in a patient’s susceptibility to further disease. An example of this is the comorbidity of allergic conjunctivitis and dry eye, and it’s of particular interest to us. Patients with chronic allergic disease experience elevated levels of immune cells and pro-inflammatory mediators, setting the stage for a protracted state of allergic inflammation that shares many of the features of dry eye.
Conversely, ocular surface abnormalities in dry eye may predispose to ocular allergy since barriers to allergen entry into conjunctival tissue are compromised. In a recent clinical study,29 experimentally induced dry eye predisposed patients to more severe responses to antigen, demonstrating the role of innate immunity in the regulation of adaptive immune responses in the eye.
It has been suggested that PRR activation and crosstalk in tissues such as the cornea may be a key in the balance between Th1 and Th2 adaptive immune responses,30 and so could represent a uniquely positioned target for therapeutic intervention. Increased understanding and appreciation of innate immunity has solidified the notion that a balanced ebb and flow of inflammatory responses to environmental stimuli is a key part of healthy tissue homeostasis. Yale researcher Ruslan Medzhitov, PhD, has proposed the term “para-inflammation” to describe a controlled physiological response that is beneficial in terms of protection from infection and maintenance of tissue and organ function.31 The broad spectrum that inflammatory mechanisms encompass is brought into perspective when considering these concepts. Maintenance of health and tissue homeostasis is a delicate balance to maintain, since organisms are constantly exposed to exogenous and endogenous stress and consequent danger signals.
Recent efforts to target the function of PRRs as a therapeutic intervention strategy have focused on TLRs (especially TLR-4, -7 and -9), and although the results from these studies have been equivocal, a number of new trials are either under way or in the planning stages. NLRs have also been the subject of great interest, both because of their importance in mediating adjuvant effects for vaccines, and as therapeutic targets in their own right. For example, the IL1-receptor antagonist Anakinra showed efficacy against a rare disease linked to NLRP3 mutation, Muckle-Wells syndrome. Trials of this agent for several other conditions known to involve NLRP3 dysfunction (familial cold urticaria and gout) also demonstrated significant efficacy.32 This is an example of how elucidation of underlying mechanisms can suggest new therapies whose utility would not otherwise be apparent.
Targeting PRR signaling has great potential in a number of ocular diseases, including conditions involving ocular surface inflammation,3 uveitis14 and retinal degenerative diseases.16 While we are still building on the foundation established long ago by Celsus, the expanded list of potential therapeutic targets generated by recent discoveries in immunity research may provide the tools to answer these questions. With these tools, there is substantial reason for optimism that future novel therapies will act to re-establish the homeostatic balance of our innate immune system.
REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School. Dr. Gamache is director of ophthalmic pharmaceuticals market research at Market Scope, LLC. Dr. McLaughlin is a medical writer at Ora Inc in Andover.
1. Medzhitov R, Janeway C. Innate Immunity. NEJM 2000;343:338-344.
2. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 2010;140:805-820.
3. Pearlman E, Sun Y, Roy S et al. Host defense at the ocular surface. International Reviews of Immunology 2013;32:4-18.
4. Sonawane S, Khanolkar V, Namavari A, et al. Ocular Surface Extracellular DNA and Nuclease Activity Imbalance: A New Paradigm for Inflammation in Dry Eye Disease. Invest Ophthalmol Vis Sci 2012;53:8253-8263.
5. Anderson, KV, Jurgens G, Nusslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell 1985;42:779-789.
6. Agostino M, Yuriev E, Ramsland PA. A Computational Approach for Exploring Carbohydrate Recognition by Lectins in Innate Immunity. Front Immunol 2011;2: 23.
7. Szabo A, Rajnavolgyi E. Collaboration of Toll-like and RIG-I-like receptors: tRIGgering antiviral innate immune responses. Am J Clin Exp Immunol 2013;2:3:195–207.
8. Kumar MV, Nagineni CN, Chin MS, Hooks JJ, Detrick B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol 2004;153:7-15.
9. Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf 2008;6:108-116.
10. Novak N, Koch S, Allam JP, Bieber T. Dendritic cells: Bridging innate and adaptive immunity in atopic dermatitis. J Allergy Clin Immunol 2010;125:50-9.
11. Kastner DL, Aksentijevich I, Goldbach-Mansky R. Auto-inflammatory disease reloaded: A clinical perspective. Cell 2010; 140:784-790.
12. Dinarello CA. Anti-inflammatory agents: Present and future. Cell 2010;140:935-950.
13. Miceli-Richard C, Lesage S, Rybojad M, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001; 29:19-20.
14. Forrester JV. Bowman lecture on the role of inflammation in degenerative disease of the eye. Eye 2013;27:340-352.
15. Erdinest N, Aviel G, Moallem E, et al. Expression and activation of toll-like receptor 3 and toll-like receptor 4 on human corneal epithelial and conjunctival fibroblasts. Journal of Inflammation 2014; 11:3.
16. Whitcup SM, Sodhi A, Atkinson JP, Holers VM, Sinha D, Rohrer B, Dick AD. The role of the immune response in age-related macular degeneration. Int J of Inflammation 2013; 1-10.
17. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331-342.
18. Rosenbaum JT, Kim HY. Innate immune signals in autoimmune and autoinflammatory uveitis. International Reviews of Immunology 2013;32:68-75.
19. Serra AM, Waddell J, Manivannan A, Xu H, Cotter M, Forrester JV. CD11b+ bone marrow-derived monocytes are the major leukocyte subset responsible for retinal capillary leukostasis in experimental diabetes in mouse and express high levels of CCR5 in the circulation. Am J Pathol 2012;181:719-727.
20. Gorin MB. Genetic insights into age-related macular degeneration: Controversies addressing risk, causality, and therapeutics. Mol Aspects of Med 2012;33:467-486.
21. Williams MA, Craig D, Passmore P, Silvestri G. Retinal drusen: Harbingers of age, safe havens for trouble. Age Ageing 2009;38:648-654.
22. Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol 2010;94:918-925.
23. Belmonte C, Gallar J. Cold thermoceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci 2011;52:888-3892.
24. Lee HS, Hattori T Park EY, Stevenson W, Chauhan SK, Dana R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci 2012;53:5632-5640
25. Amparo F, Dastjerdi MH, Okanobo A, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: A randomized clinical trial. JAMA Ophthalmol 2013;6:715-723.
26. Li DQ, Zhang L, Pflugfelder SC, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphoprotein/OX40 ligand/OX40 signaling pathways. J Allergy Clin Immunol 2011;128: 1318.
27. Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nature Immunology 2010;11:289-293.
28. Sandig H, Bulfone-Paus S. TLR signaling in mast cells: Common and unique features. Frontiers Immunology 2012;3:185-193.
29. Gomes PJ, Ousler GW, Welch DL, Smith LM, Coderre J, Abelson MB. Exacerbation of signs and symptoms of allergic conjunctivitis by a controlled adverse environment challenge in subjects with a history of dry eye and ocular allergy. Clinical Ophthalmology 2013;7:157-165.
30. Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511-9.
31. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:7203:428-435.
32. Geddes K, Magalhães JG Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov 2009;8:465-479.