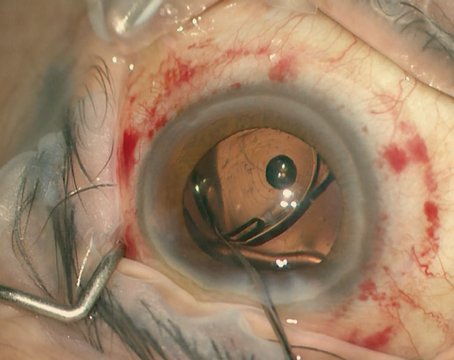
When John R. Campbell, MD, and David F. Chang, MD, noted that certain cataract patients in their respective California practices experienced iris billowing, progressive pupil constriction and iris prolapse during surgery, they investigated. A retrospective review and a larger, prospective companion study published in 2005 led to the first description of intraoperative floppy iris syndrome.1 Here’s a look at where we are in terms of awareness of IFIS across specialties, and an overview of prevention and management techniques.
That 2005 study implicated the use of tamsulosin (Flomax; Boehringer Ingelheim; Ridgefield, Connecticut) an alpha-1 adrenergic antagonist used to treat benign prostate hyperplasia (BPH) in men. Tamsulosin has other uses too, including relaxing the smooth muscles in the bladder for women who have difficulty passing urine, facilitating the release of kidney stones and treating hypertension. Other alpha-blockers used for BPH therapy include erazosin, doxazosin, alfuzosin and silodosin.
“It also causes changes to the receptors that are responsible for contraction of the iris sphincter muscles,” says Steven M. Silverstein, MD, of Silverstein Eye Centers in Kansas City, Missouri, of tamsulosin (and other alpha blockers). “Urology is the primary specialty that prescribes that class of drugs. Primary care, including internal and family medicine, do as well. And to a lesser degree, even OB-GYN specialists prescribe these medicines.”
Alpha-Blockers
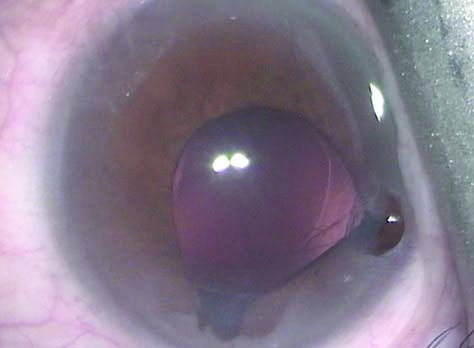
As many as 80 to 90 percent of men develop BPH by their 70s or 80s.2 This is important because IFIS complicates cataract surgery to varying degrees, potentially leading to iris trauma, posterior capsular tears and vitreous loss. The classic signs of iris billowing, pupil constriction and iris prolapse are collectively referred to as the IFIS triad. When the patient’s history of taking tamsulosin or even other, less-selective alpha antagonists is known, however, surgeons can factor IFIS risk into their plans and deliver good outcomes. Getting a positive history of alpha-blocker use may not be that simple, however. Even one dose of tamsulosin seems to predispose patients to IFIS forever, so discontinuing treatment appears to be useless for mitigating IFIS risk;3 and a patient may not remember a long-discontinued medication.
“The only real way to prevent IFIS is to avoid using this class of medicines in patients who haven’t yet undergone cataract surgery,” says Dr. Silverstein. “If you’ve taken the medicine as briefly as just two or three days and then discontinued it, you still have the potential for IFIS complications for the rest of your life. There’s no benefit in terms of decreasing risk by stopping the medication prior to surgery.”
Poor pupil dilation with mydriatic drop instillation during a preop workup is predictive of severe IFIS risk,3 but surgeons should still strive to get a definitive history regarding the patient’s use of tamsulosin and other alpha antagonists. To that end, the American Academy of Ophthalmology and the American Society of Cataract and Refractive Surgery have issued joint advisories on alpha-blockers for prescribing providers and patients. The latest educational update for providers, from 2014, urged them to consider initiating therapy with a less-selective substitute for tamsulosin, based on research showing that one such alternative alpha-blocker, alfusozin, was associated with a significantly lower incidence of severe IFIS than tamsulosin.4 The update also advised that cataract patients slated for non-emergent tamsulosin therapy may wish to undergo cataract surgery prior to initiating the drug treatment. For patients, the AAO and ASCRS advise those with cataracts to consider the relationship between alpha-blocker use and increased risk of cataract-surgery complications before initiating drug therapy; caution patients taking alpha-blockers to inform their ophthalmologist prior to ocular surgery; and advise cataract patients who need alpha-blocker therapy to consider starting with a nonselective alpha antagonist instead of tamsulosin. The advisory also suggests that patients with symptomatic cataracts consider undergoing cataract surgery prior to starting any alpha-blocker treatment.
“From a primary-care, urology and OB-GYN standpoint, providers should check with the patient to see whether or not the patient and their ophthalmologist are contemplating cataract surgery in the near future,” says Dr. Silverstein. “If so, they should hold off on prescribing these medicines until after cataract surgery whenever possible.” He believes that awareness is growing among prescribing providers. “I would estimate that at this point, probably two-thirds of the providers prescribing alpha-1 antagonists are aware of the potential risks to the eye with surgery in patients who take these medicines,” he says.
Pharmacological Prophylaxis
Intracameral mydriatic agents were found to be safe and effective prior to the identification of IFIS.5 To prevent IFIS and its signs, Samuel Masket described pre-medicating at-risk patients with topical atropine sulfate 1% three times a day for two days before surgery, standard mydriatics and intraoperative intracameral epinephrine, attributing success in 19/20 eyes to synergy between the pupillary block provided by atropine and the iris-dilator stimulation of the epinephrine.6 The late Joel K. Shugar, MD, MSEE, subsequently described using intracameral epi-shugarcaine (9 cc fortified BSS; 4 cc bisulfite-free 1:1000 epinephrine, and 3 cc preservative-free lidocaine) and eliminating preoperative atropine.7 Intracameral phenylephrine has also been used to prevent IFIS.8
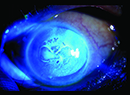
 |
| Iris prolapse signifies severe IFIS progressing from iris billowing and miosis. |
In 2017, the Food and Drug Administration issued an advisory letter to health-care providers warning that Par Pharmaceuticals, an important generic supplier of bisulfite-free epinephrine, had changed its formulation, adding tartaric acid, making it unsafe for intraocular use. In December 2017, the AAO and ASCRS both advised surgeons of the shortage. Among the coping strategies the ASCRS recommended were seeking preservative-free epinephrine from small suppliers; substituting phenylephrine and lidocaine and considering the use of Omidria (Omeros; Seattle) phenylephrine 1% ketorolac 0.3% as an alternative.
Dr. Silverstein has adopted Omidria in the irrigation bottle for cataract cases, regardless of known IFIS risk factors. “Intraoperatively, it’s the only thing I use to decrease the risk of IFIS in a majority of cases,” he says. Dr. Silverstein was the first author on a study comparing men with a history of tamsulosin use who had Omidria in the irrigation bottle with those who didn’t have it. The treated group had significantly less miosis, pupil billowing and iris prolapse during cataract surgery than tamsulosin-treated men who did not.9 “Its use showed a statistically significant reduction in the triad of signs and symptoms of IFIS—mainly iris billowing and flaccidity, pupillary miosis and iris prolapse—compared to those who did not receive treatment,” he says.
Mechanical Measures
Although IFIS prevention and management isn’t standardized, a menu of accepted interventions exists. “I’m thankful to David Chang and John Campbell for doing the original epidemiology that brought attention to and awareness of the relationship between alpha-1 antagonists and IFIS,” says Dr. Silverstein. “With this awareness comes a variety of solutions we can employ.”
If mydriatic and/or pupilloplegic drugs don’t prevent IFIS signs, iris hooks and pupillary rings can help keep the pupil big enough for phaco to safely continue; each has its benefits. “Every case is unique and there are times you may use a pupil-expansion device such as a Malyugin ring or iris hooks,” Dr. Silverstein observes. “Sometimes, you can use a heavier molecular-weight OVD to help hold the iris back during phacoemulsification. I prefer the Malyugin ring for its ease of use, but iris hooks are more cost-effective since they can be sterilized and reused,” he says.
Further modifying surgical techniques may also prove helpful. A small study suggests that the creation of an additional incision near the original wound in the event of iris prolapse can provide as a secure place for the iris to go, or it can accommodate the phaco tip so that the procedure can continue.10
Whatever measure or measures the surgeon employs to prevent and manage IFIS, being forewarned helps set the stage for good outcomes.11 A 2016 study indicated that a knowledge gap in the practice patterns of urologists when it comes to the ophthalmic risks of BPH medications remains, however.12 The researchers received 175 responses to a questionnaire emailed to all of the urology residency programs in the United States, and all the members of the Western section of the American Urological Association. Twenty-one percent of respondents never asked patients about their ophthalmic health before initiating BPH treatment; 37 percent would routinely encourage patients with complaints about their vision to consult an ophthalmologist prior to starting BPH treatment; just 13 percent would routinely refer such patients to an ophthalmologist. Residents were significantly less likely to ask BPH patients about visual complaints or refer those with such complaints to an ophthalmologist prior to starting therapy than were fellows and attendings (p<0.01).
“Urologists and urology health-care providers need more education and awareness in the form of CME-type lectures, presentations, publications and inclusion in residency curriculums,” says one of the study’s authors, Sia Daneshmand, MD, associate professor of urology and director of the urologic oncology fellowship program at the USC/Norris Comprehensive Cancer Center. REVIEW
Dr. Silverstein reports no financial ties to the products discussed in this article.
1. Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
2. Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev Urol 2005;7:Suppl 9: S3–S14.
3. Chang DF, Braga-Mele R, Mamalis N et al. ASCRS white paper: Clinical review of intraoperative floppy-iris syndrome. J Cat Refract Surg 2008;34:2153-62.
4. Chang DF, Campbell JR, Colin J et al. Prospective masked comparison of intraoperative floppy iris syndrome severity with tamsulosin versus alfuzosin. Ophthalmol 2014;121:4:829–34.
5. Lundberg B, Behndig A Intracameral mydriatics in phacoemulsification cataract surgery. J Cataract Refract Surg 2003;29:12:2366-71.
6. Masket S, Belani S. Combined preoperative topical atropine sulfate 1% and intracameral nonpreserved epinephrine hydrochloride 1:4000 [corrected] for management of intraoperative floppy-iris syndrome. J Cataract Refract Surg 2007;33:4:580-2.
7. Shugar JK. Use of epinephrine for IFIS prophylaxis. J Cataract Refract Surg. 2006;32:7:1074-1075.
8. Gurbaxani A, Packard R. Intracameral phenylephrine to prevent floppy iris syndrome during cataract surgery in patients on tamsulosin. Eye 2007;21: 331–332.
9. Silverstein SM, Rana VK, Stephens R, et al. Effect of phenylephrine 1.0%-ketorolac 0.3% injection on tamsulosin-associated intraoperative floppy-iris syndrome. J Cataract Refract Surg 2018;44:9:1103-08.
10. Simaroj P, Lekhanont K, Charukamnoetkanok P. Modified surgical technique for managing intraoperative floppy iris syndrome. Case Rep Ophthalmol Med 2016; Article ID 1289834. http://dx.doi.org/10.1155/2016/1289834.
11. Chang DF, Osher RH, Wang L, Koch DD. A prospective multicenter evaluation of cataract surgery in patients taking tamsulosin (Flomax). Ophthalmol 2007;114:957-964.
12. Zhang Y, Shamie N, Daneshmand S. Assessment of urologists’ knowledge of intraoperative floppy iris syndrome. Urology 2016;97:40-5.