In clinical study of 149 eyes treated using the iDESIGN system, 91.9 percent of all eyes achieved uncorrected visual acuity of 20/20 or better without glasses at three months postop.
The iDESIGN system controls the procedure by generating a high-definition scan that measures and maps the irregularities of the eye that may impact visual acuity. Abbott says that iDESIGN then creates an accurate and personalized treatment plan based on these measurements.
The new approval allows for use in patients with mixed astigmatism measured by the iDESIGN system in which the magnitude of cylinder (1 to 5 D) is greater than the magnitude of sphere; who are 18 years of age or older; and who have stable refractions (a change of less than 1 D in sphere or cylinder for a minimum of 12 months prior to surgery).
For more information on Abbott’s iDESIGN system, visit vision.abbott/us/.
FDA Approves Icare HOME
Icare USA recently announced that the Icare HOME tonometer has been approved by the FDA and is now available for use in the United States. Icare claims that the recent clearance will give doctors the ability to monitor their patients with more regularity and confidence, as patients are now able to measure their own IOP. Doctors can study IOP fluctuations throughout the day.
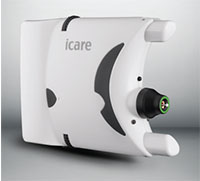 |
Icare highlights the ease of use for patients. The unit’s Icare EyeSmart technology performs automatic OD/OS recognition. The company also says that the positioning is simple, thanks to red and green light signals that help patients correctly orient the tonometer. The Icare HOME also comes equipped with an automated measuring sequence that can take either a single measurement or a series of six.
Icare says that since the Icare HOME involves no puff of air and requires no drops, it’s very convenient for patients to use.
For more information on the Icare HOME, visit icare-usa.com.
CooperVision’s Clariti One-day Toric Contacts
In early March, CooperVision announced the release of a 90-pack configuration for its clariti one-day toric contact lenses. It says that these lenses provide astigmatic patients with the advantages of silicone hydrogel and the new convenience of one-day use. The clariti one-day toric is the only silicone hydrogel, one-day lens for astigmatism broadly available in the United States, the company states.
CooperVision also says these silicone hydrogel soft contact lenses feature high water content to support all-day comfort. It adds that the clariti one-day lenses have high oxygen transmissibility, allowing for 100 percent corneal oxygen consumption, which promotes ocular health.
The toric lens’s power ranges include: plano to -9 D with cylinder options of -0.75 D, -1.25 D and -1.75 D in axes of 10, 20, 60, 70, 80, 90, 100, 110, 120, 160, 170 and 180 degrees; +0.25 D to +4 D with cylinder options of -0.75 D, -1.25 D and -1.75 D in axes of 20, 70, 90, 110, 160 and 180 degrees; and plano to -9 D with a cylinder power of -2.25 D in axes of 10, 20, 90, 160, 170 and 180 degrees.
For more information, visit coopervision.com.



