 |
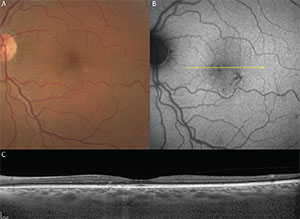
Figure 1. Pachychoroid pigment epitheliopathy in a 61-year-old. A. Color fundus image. B. Fundus autofluorescence. C. Structural optical coherence tomography. |
The pachychoroid spectrum exemplifies our ability to harness these new modalities in order to understand better the choroidal changes that underlie central serous chorioretinopathy and to define an expanded phenotype through recognition and classification of several macular disorders that share a similar choroidal phenotype.
Features
The pachychoroid phenotype features: 1) reduced fundus tessellation on clinical examination or white light photography; 2) relatively increased choroidal thickness, which may be focal or diffuse; 3) pathological dilation of outer choroidal (Haller) vessels, referred to as “pachyvessels”; and 4) loss of choriocapillaris and Sattler layers overlying pachyvessels.3,4 Pachyvessel morphology has been further characterized by en face structural OCT, which also provides valuable data on the topological variations in choroidal thickness.2
Pachychoroid pigment epitheliopathy (PPE) refers to a precursor or forme fruste of CSC in which focal or multifocal RPE changes are seen without evidence of concurrent or preceding neurosensory detachment.3 The RPE changes occurring in PPE resemble those frequently detected in the fellow eye of patients presenting with unilateral neurosensory detachments due to CSC. Like those with CSC, eyes with PPE also exhibit choroidal hyperpermeability on indocyanine green angiography co-localized with pachychoroid features.5 Failure to recognize these choroidal changes can result in these patients being assigned other diagnoses such as early AMD, atypical pattern dystrophy, retinal pigment epitheliitis or punctate inner choroidopathy (See Figure 1).
The location and distribution of RPE changes in PPE are spatially co-localized with the distribution of pachyvessels, particularly where they lie in close proximity to the Bruch’s membrane-RPE complex, due to focal loss of the overlying choriocapillaris and Sattler layer volume.2
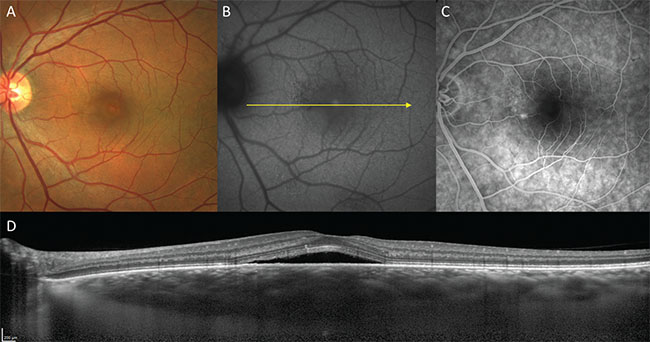 |
| Figure 2. Acute central serous chorioretinopathy in a 55-year-old male. A. Color fundus image. B. Fundus autofluorescence. C. Fluorescein angiography. D. Structural optical coherence tomography. |
Central serous chorioretinopathy is a well-described disorder in which serous pigment epithelial detachments and serous neurosensory detachments occur at the posterior pole in the absence of inflammatory features.6 Subretinal fluid accumulates via focal or diffuse leaks at the level of the RPE, which are demonstrable by fluorescein angiography, and is therefore thought to originate in the choroid. OCT shows the smooth, convex profile typical of serous pigment epithelial detachments and that the subretinal fluid is often detected above a serous PED showing leakage on FA (See Figure 2).
In the acute form, CSC is usually self-limited with good visual recovery. However, a subset of patients may experience either a chronic or remitting-relapsing course with a range of complications and compromised visual acuity. Chronicity is often characterized by RPE changes occurring in the distribution of subretinal fluid. These RPE changes may be non-specific or may have a gravitating morphology that is best visualized on fundus autofluorescence.7 OCT may also show outer retinal changes, such as thickened photoreceptor outer segments and ellipsoid zone disruption associated with chronic subretinal fluid. Complications of chronic CSC may include outer retinal atrophy, neovascularization, cystic macular degeneration and bullous retinal detachment.8
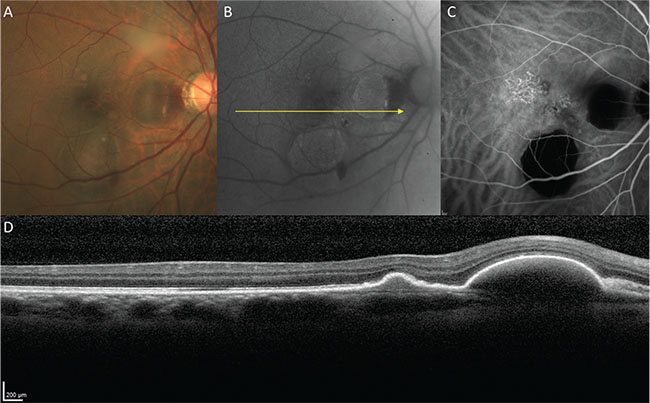 |
| Figure 4. Polypoidal choroidal vasculopathy in a 58-year-old male. A. Color fundus image. B. Fundus autofluorescence. C. Indocyanine green angiography (mid-phase). D. Structural optical coherence tomography. |
Pachychoroid Neovasculopathy
Pachychoroid neovasculopathy (PNV) refers to the presence of type 1 neovascularization in eyes with a pachychoroid phenotype.4 Patients developing PNV are typically a decade or two younger than patients developing neovascular age-related macular degeneration. Pachychoroid neovasculopathy also differs from neovascular AMD in that it occurs in eyes with thicker choroids and pachyvessels with absent or minimal drusen. Type 1 neovascularization in PNV often develops insidiously and may arise in eyes with PPE which have not previously exhibited frank subretinal fluid (See Figure 3).
In PNV, hyperfluorescence on FA due to type 1 neovascularization may be ill-defined and difficult to detect when it occurs within a background of RPE changes related to chronic CSC. With ICGA, late-staining plaques representing type 1 neovascularization may be obscured from detection by choroidal hyperpermeability. Cross-sectional OCT is often helpful in showing findings suggestive of sub-RPE neovascularization including shallow irregular RPE elevations containing material of heterogeneous but intermediate reflectivity. These shallow, irregular PEDs have frequently been shown by OCT angiography to harbor neovascular tissue. The finding of shallow irregular PEDs in pachychoroid eyes on tsructural OCT is therefore considered to be a sensitive sign for neovascularization.
Polypoidal choroidal vasculopathy was first described by Lawrence Yannuzzi, MD, and associates in terms of polypoidal vascular lesions, thought to occur in the inner choroid, that predispose to exudative and hemorrhagic complications.9 In the initial description, PCV was described by the ICGA appearance of the polypoidal lesions. Subsequent studies with OCT localized both the polypoidal lesions and their feeding vascular networks below the RPE, but above Bruch’s membrane, suggesting that polypoidal lesions originate from type 1 neovascular tissue10 (See Figure 4).
The factors predisposing to the development of polypoidal lesions in some patients with type 1 neovascularization remain unclear, but the fact that PCV occurs with greater frequency in patients with Asian and African ancestry suggests some genetic basis. Although PCV is traditionally studied and treated as a variant of AMD, it often occurs in eyes that lack drusen and in patients who are younger than those with typical AMD. Additionally, in both Asian and Caucasian patients with polypoidal lesions, cross-sectional and en face OCT frequently show choroidal features more consistent with the pachychoroid phenotype than with AMD.2 Patients with a pachychoroid phenotype, type 1 neovascularization and associated polypoidal lesions might be better classified as having a pachychoroid spectrum entity than a variant of AMD.11
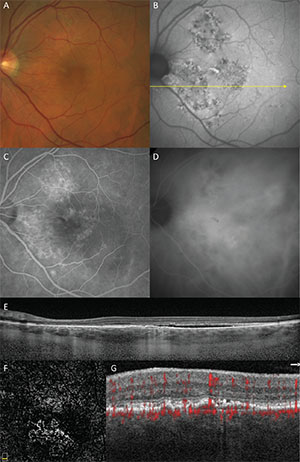 |
| Figure 3. Pachychoroid neovasculopathy in a 65-year-old male. A. Color fundus image. B. Fundus autofluorescence. C. Fluorescein angiography (late phase). D. Indocyanine green angiography (late phase). E. Structural optical coherence tomography. F. OCT angiography segmented through the shallow irregular pigment epithelial detachment. G. Detail of cross-sectional OCT angiography with flow signal through the shallow irregular pigment epithelial detachment. |
In summary, multimodal imaging has enabled an enhanced appreciation of specific choroidal features associated with CSC and an expanded spectrum of macular disease including PPE, PNV and a subset of PCV. Previously, patients presenting with these entities were difficult to diagnose (PPE), or classified as having a variant of AMD.12 The definition of the pachychoroid phenotype continues to evolve as we sub-segment the choroid using novel imaging modalities and tools. With an enhanced appreciation regarding the significance of pathologic choroidal changes in a variety of macular diseases, research may now expand to explore new disease mechanisms with potential impact on therapeutic strategies and visual outcomes. REVIEW
Dr. Dolz-Marco is an international medical retina fellow at Vitreous Retina Macula Consultants of New York and junior researcher at Unit of Macula, Institute of Health Research, University and Polytechnic Hospital La Fe in Valencia, Spain. Dr. Dansingani is an assistant professor of ophthalmology and visual sciences at Truhlsen Eye Institute, University of Nebraska Medical Center, in Omaha, Neb. Dr. Freund is a retina specialist at Vitreous Retina Macula Consultants of New York; clinical professor of ophthalmology at the New York University School of Medicine; and on staff at New York Presbyterian Hospital, Manhattan Eye Ear & Throat Hospital, and Lenox Hill Hospital. Contact Dr. Freund at kbfnyf@aol.com.
1. Mrejen S, Spaide RF. Optical coherence tomography: Imaging of the choroid and beyond. Surv Ophthalmol 2013;58(5):387-429.
2. Dansingani KK, Balaratnasingam C, Naysan J, Freund KB. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 2016;36:499-516.
3. Warrow DJ, Hoang QV, Freund KB. Pachychoroid pigment epitheliopathy. Retina 2013;33:1659-1672.
4. Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina 2015;35:1-9.
5. Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol 2014;158(2):362-371 e362.
6. von Graefe A. Ueber centrale recidivierende retinitis. Graefe’s Arch Clin Exp Ophthalmol 1866;12:211-215.
7. Imamura Y, Fujiwara T, Spaide RF. Fundus autofluorescence and visual acuity in central serous chorioretinopathy. Ophthalmology 2011;118:700-705.
8. Sahu DK, Namperumalsamy P, Hilton GF, de Sousa NF. Bullous variant of idiopathic central serous chorioretinopathy. Br J Ophthalmol 2000;84(5):485-492.
9. Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990;10:1-8.
10. Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995;15:100-110.
11. Balaratnasingam C, Lee W, Koizumi H, Dansingani K, Inoue M, Freund K. Polypoidal choroidal vasculopathy: A distinct disease or manifestation of many? Retina 2016;36:1-8.
12. Gallego-Pinazo R, Dolz-Marco R, Gómez-Ulla F, Mrejen S, Freund KB. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 2014;3(4):111-115.



