Without question, corticosteroids—parenteral, regional or topical—are the mainstay of therapy for uveitic CME. But when disease remains refractory to these standard therapies, or they’re contraindicated (e.g., in advanced glaucoma or in patients known to develop ocular hypertension in response to corticosteroids), finding effective alternatives can be challenging. Intravitreal anti-VEGF agents, though temporarily effective in some cases,6,7 aren’t a practical option for chronic cases, especially in the setting of bilateral disease, given the rapid recrudescence of fluid and need for frequent, and perhaps indefinite, injections.8-10 The following brief review discusses some of the more promising systemic therapies for precisely this indication: chronic, inflammatory CME for which conventional therapy has failed or carries excessive risk.
Systemic Interferons
Given its rapidity of effect and near complete response rate, subcutaneously (SC) injected interferon alpha should be considered early in the treatment algorithm for chronic, uveitic CME once it’s been determined that corticosteroids are contraindicated or ineffective. This is especially true for bilateral and severe disease, as the benefits must significantly offset the numerous risks and adverse events associated with this therapy. While the precise mechanism of action regarding its efficacy for CME remains elusive, investigators have demonstrated that interferon alpha-2b decreases the permeability of bovine retinal microvasculature.11
The use of interferon for ocular inflammation began with Behçet’s disease patients;12 indeed, Behçet himself had suspected a viral etiology for this disease as early as 1937.13 In 2003, Germany’s Ina Kötter, MD, and colleagues14 prospectively enrolled 50 Behçet’s disease patients with refractory uveitis and demonstrated a 92-percent response rate to SC injected interferon alpha-2a. Incidentally, in 58 eyes with angiographic CME, they noted 100-percent resolution. Separately, in a small trial of 12 patients with intractable posterior or panuveitis, only two of whom had Behçet’s disease, uveitis significantly improved in 10 patients with subcutaneous interferon alpha-2b, and in the 14 eyes with CME the authors noted rapid resolution in all cases.15
Encouraged by the prompt and complete resolution of CME seen in their prior trial of 50 Behçet’s disease patients, the same investigators designed a pilot study to assess the efficacy of systemic interferon alpha for uveitic CME in patients without associated Behçet’s disease.16 In all 15 eyes (of eight patients), the uveitis was quiescent and the CME remained refractory and long-standing (mean duration: 52 months). Thirteen of 15 eyes demonstrated 100-percent resolution of CME within two to four weeks of starting interferon alpha-2a, 3 or 6 million units SC daily (mean foveal thickness change: 551 to 143 µm; mean best-corrected visual acuity gain: +0.80 to +0.42 logMAR). Later, they expanded this consecutive, interventional case series to 24 patients (40 eyes) and reported on them retrospectively, finding interferon “effective” in 62.5 percent of patients and partly effective in 25 percent.17 Underscoring the stringency of their outcome measure definitions, “partly effective” eyes still experienced more than a 300-µm reduction in mean foveal thickness by the end of follow-up (587 to 285 µm), and roughly half of the eyes in each group—“effective” and “partly effective”—gained three or more lines during follow-up.
In close agreement with these findings, a study in which I took part demonstrated 100-percent resolution of uveitic CME in eight eyes (four patients).18 In all cases, the CME was long-standing (mean duration: 31 months) and refractory to numerous other therapies, and the response to interferon alpha-2b SC was rapid and clinically meaningful (mean BCVA gain: 20/129 to 20/56, p=0.0004; mean central macular thickness change: 563 to 267 µm, p=0.002). Most recently, in a randomized controlled trial of 48 patients, interferon alpha, comparable to the systemic corticosteroid arm, effected a significant reduction in CMT relative to an untreated control group.19 These differences between treated and untreated groups didn’t meet statistical significance criteria in the intention-to-treat analysis—only in the per-protocol analysis—likely because the study failed to complete its projected enrollment.
Aside from the clear indication for Behçet’s disease-associated uveitis and CME, systemic interferon may be a particularly apt therapy in several other clinical situations. For patients with multiple sclerosis, in addition to treating the underlying disease, interferon beta has demonstrated efficacy for MS-associated uveitis and uveitic CME.20 An additional case scenario involves the treatment of chronic, inflammatory CME in the setting of prior intraocular infection, in which intravitreal or systemic corticosteroid may put the patient at risk of relapse.21,22 Systemic interferon carries no such risk; in fact, in the setting of quiescent viral infections (e.g., cytomegalovirus retinitis or acute retinal necrosis), interferon offers a protective antiviral effect. Lastly, interferons don’t carry a risk of malignancy; on the contrary, they have antiproliferative effects, and therefore may be particularly suitable for chronic CME in the setting of autoimmune or cancer-associated retinopathy.
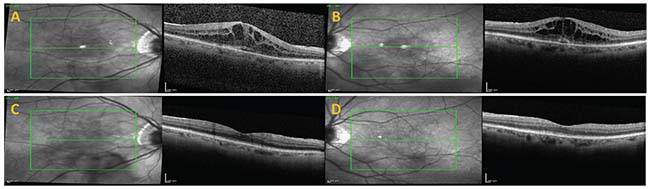 |
Exogenous interferon therapy has numerous side effects and associated adverse events, but they are rarely fatal or life-threatening.23 Virtually all patients will develop a flu-like illness. Other important adverse events include neutropenia, thrombocytopenia, liver transaminase elevations, suicidality and exacerbation of underlying autoimmunity.24 With careful monitoring, however, cessation of therapy is rarely required.
Tocilizumab
More recently, the interleukin-6 (IL-6) receptor blocker tocilizumab has been gaining traction as a niche therapy for uveitic CME, based initially on rather modest results from a small case series.25 The rationale for this approach stems from our understanding of the pro-inflammatory nature of IL-6 and its increased expression in the ocular fluid of patients with uveitis26 and macular edema from various causes.27,28 Following the initial report, separate investigators demonstrated dramatic improvement in CMT (896 to 182 µm) in a single patient with panuveitis refractory to multiple therapies, including tumor necrosis factor alpha (TNF-α) blockade, after commencing monthly infusions of tocilizumab.29 Bolstered by the results of their test case, the same group continued to employ anti-IL-6 therapy in the setting of recalcitrant inflammatory CME and retrospectively reported their experience with five consecutive patients.30 Prior to tocilizumab, the uveitis was quiescent in all cases but the CME persisted, despite therapy with multiple immunosuppressive and biologic agents. Mean CMT improved with anti-IL-6 therapy by more than 200 µm from baseline to month one (p=0.006). By six months of follow-up, mean CMT had reduced by more than 300 µm from baseline and half of eyes (p=0.028) had gained ≥ 2 lines of BCVA. The same investigators next demonstrated that the beneficial effects of tocilizumab for uveitic CME extend out to 12 months in a retrospective cohort study of seven patients; though they also discovered that CME rapidly recurred in the two patients in whom they attempted to discontinue therapy.31 Recently, the 24-month results for this same cohort of patients, expanded to 12 (16 eyes), have been published, confirming that uveitic CME remains controlled out to two years, but rapidly recrudesces with suspension of therapy. Five out of five patients relapsed within one to three months of stopping tocilizumab.32
Others have repeated these results, finding similar efficacy for IL-6 blockade with inflammatory macular thickening in the setting of specific uveitic disease entities, such as juvenile idiopathic arthritis (JIA)33,34 and birdshot chorioretinititis.35 All of the enrolled patients in these studies had intractable disease, having failed multiple prior therapies, including at least one TNF-α inhibitor, suggesting that tocilizumab may be even more effective in selected patients. In assessing five patients (eight eyes) with similarly severe uveitis, the German group responsible for much of the work regarding type-I interferons for uveitic CME found at least a 25-percent reduction in CMT with tocilizumab in 75 percent of eyes.36 The systemic disease associations, though not uniform, were more homogenous (JIA (in two patients), rheumatoid arthritis (2), ankylosing spondylitis (1) and all had active inflammatory arthritis, which was the main reason the investigators avoided recombinant interferon alpha.
Overall, tocilizumab, in comparison with systemic interferon, appears to clear inflammatory CME less rapidly but with significantly fewer side effects and adverse events. Importantly, whereas exogenous interferons may be associated with triggering or exacerbating underlying autoimmunity, IL-6 blockade in many cases may be an effective treatment for uncontrolled inflammation (in the eye or elsewhere in the body). Thus, the patient’s systemic disease activity should be taken into account when considering this therapy for uveitic CME.
Octreotide
Much less evidence exists in support of octreotide, a somatostatin analog, for chronic, uveitic CME. The available literature, however, does suggest efficacy. In 1998, a single case report linked octreotide therapy with resolution of long-standing, refractory, idiopathic CME and suggested that this treatment might have benefit for macular edema from other causes.37 In 2005, Greece’s Thekla Papadaki, MD, and her colleagues provided the first evidence that octreotide may have potential for treating uveitic CME.38 After failing standard therapy (oral acetazolamide, topical and systemic non-steroidal anti-inflammatory drugs, and regional steroid injections), the patient’s bilateral CME responded completely to octreotide 100 µg SC t.i.d. Notably, her uveitis, prior to initiation of octreotide, had been under complete control with methotrexate. Following this report, the same group published results of five patients (nine eyes) with uveitic macular edema resistant to standard therapy, finding “marked improvement or complete resolution” of fluid in seven of nine eyes.39 One patient with bilateral edema had no response in either eye. For the entire cohort, the mean foveal thickness improved from 496 to 241 µm over a range of eight to 24 months of treatment. In the largest study to date, analyzing 20 patients with quiescent uveitis, investigators found that monthly intramuscular injection of a long-acting formulation of octreotide decreased CME significantly in 70 percent of episodes.40
The mechanism of action for octreotide’s effect on CME is poorly understood. Somatostatin and its analogs inhibit insulin-like growth factor 1, a potent promoter of blood-retina barrier breakdown.41 Further, somatostatin appears to act directly at the level of the retinal pigment epithelium, enhancing its apical-basal fluid transport function.40 Investigators have determined that vitreous concentrations of somatostatin are significantly reduced in quiescent uveitis patients with chronic macular edema as compared to controls (39 pg/ml vs. 487 pg/ml; p<0.0001),42 suggesting a role for octreotide as replacement therapy in this setting. With regard to side effects, octreotide is very well tolerated, though clinicians should screen patients for symptoms of gastrointestinal distress and cholelithiasis.
ICAM Inhibitors
The anti-CD11a antibody efalizumab interferes with intercellular adhesion molecule-1 functionality. In patients with uveitis, ICAM-1 is significantly upregulated and increases vascular permeability.43,44 A single case report45 and a small case series46 suggest that efalizumab may have efficacy for patients with chronic CME. The drug was voluntarily withdrawn from the market by the manufacturer in 2009, however, due to concerns of a possible association with progressive multifocal leukoencephalopathy (PML).
Natalizumab, another adhesion molecule inhibitor, has been approved for relapsing MS and, more recently, moderate to severe Crohn’s disease. Despite also carrying an increased risk for PML, natalizumab remains available on a restricted basis when the clinical benefits outweigh the risks of therapy. As such, natalizumab may be considered in the setting of MS or Crohn’s disease with associated uveitis and sight-threatening macular edema refractory to other therapies.
In Conclusion
Unlike other complications of uveitis such as cataracts, the vision loss of uveitic CME may not be recoverable once the disease has persisted long enough to damage photoreceptors. Most patients will respond to standard therapy consisting of the escalation of immunosuppression, the possible addition of acetazolamide, and/or supplemental corticosteroid therapy. However, in some patients, the uveitis is already quiescent and corticosteroids fail (or they are contraindicated), and inflammatory CME may become chronic and persist for years, if not indefinitely. These patients gradually lose vision, and some of this vision loss may be attributable to ophthalmologists’ lack of awareness of effective alternatives. Hopefully, with increasing knowledge of the high efficacy of available treatments such as systemic interferon alpha, tocilizumab and octreotide, clinicians will be better able to control chronic, uveitic CME far earlier in the disease process, long before permanent damage occurs. REVIEW
Dr. Butler is an assistant professor of ophthalmology at Harvard Medical School, Department of Ophthalmology, Massachusetts Eye and Ear. He’s also an attending ophthalmologist at the VA Boston Healthcare System. He can be contacted at: (857) 364-4635 or nicholas.butler4@va.gov.
1. Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:4:332-6.
2. Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology 2006;113:8:1446-9.
3. Okhravi N, Lightman S. Cystoid macular edema in uveitis. Ocul Immunol Inflamm 2003;11:1:29-38.
4. Levin MH, Pistilli M, Daniel E, et al. Systemic immunosuppressive therapy for eye diseases cohort study. Incidence of visual improvement in uveitis cases with visual impairment caused by macular edema. Ophthalmology 2014;121:2:588-95.
5. Tomkins-Netzer O, Lightman S, Drye L, et al, Multicenter Uveitis Steroid Treatment Trial Research Group. Outcome of treatment of uveitic macular edema: The multicenter uveitis steroid treatment trial 2-year results. Ophthalmology 2015;122:11:2351-9.
6. Cordero Coma M, Sobrin L, Onal S, et al. Intravitreal bevacizumab for treatment of uveitic macular edema. Ophthalmology 2007;114:8:1574-79.
7. Acharya NR, Hong KC, Lee SM. Ranibizumab for refractory uveitis-related macular edema. Am J Ophthalmol 2009;148:2:303-9.
8. Mackensen F, Heinz C, Becker MD, et al. Intravitreal bevacizumab (Avastin) as a treatment for refractory macular edema in patients with uveitis: A pilot study. Retina 2008;28:1:41.
9. Bae JH, Lee CS, Lee SC. Efficacy and safety of intravitreal bevacizumab compared with intravitreal and posterior sub-tenon triamcinolone acetonide for treatment of uveitic cystoid macular edema. Retina 2011;31:1:111-8.
10. Reddy AK, Cabrera M, Yeh S, et al. Optical coherence tomography-guided ranibizumab injection for cystoid macular edema in well-controlled uveitis: Twelve-month outcomes. Retina 2014;34:12:2431-8.
11. Gillies MC, Su T. Interferon-alpha 2b enhances barrier function of bovine retinal microvascular endothelium in vitro. Microvasc Res 1995;49:3:277-88.
12. Feron EJ, Rothova A, van Hagen PM, et al. Interferon-alpha 2b for refractory ocular Behçet’s disease. Lancet 1994;343:8910::1428.
13. Behçet H, Matteson EL. On relapsing, aphthous ulcers of the mouth, eye and genitalia caused by a virus. Dermatol Wochenschr 1937;36:1152-7.
14. Kötter I, Zierhut M, Eckstein AK, et al. Human recombinant interferon alfa-2a for the treatment of Behçet’s disease with sight threatening posterior or panuveitis. Br J Ophthalmol 2003;87:4:423-31.
15. Plskova J, Greiner K, Forrester JV. Interferon-alpha as an effective treatment for noninfectious posterior uveitis and panuveitis. Am J Ophthalmol 2007;144:1:55-61.
16. Deuter CM, Koetter I, Guenaydin I, et al. Interferon alpha-2a: A new treatment option for long lasting refractory cystoid macular edema in uveitis? A pilot study. Retina 2006;26:7:786-91.
17. Deuter CM, Kötter I, Günaydin I, et al. Efficacy and tolerability of interferon alpha treatment in patients with chronic cystoid macular oedema due to non-infectious uveitis. Br J Ophthalmol 2009;93:7:906-13.
18. Butler NJ, Suhler EB, Rosenbaum JT. Interferon alpha 2b in the treatment of uveitic cystoid macular edema. Ocul Immunol Inflamm 2012;20:2:86-90.
19. Fardeau C, Simon A, Rodde B, et al. Interferon-alpha2a and systemic corticosteroid in monotherapy in chronic uveitis: Results of the randomized controlled BIRDFERON study. Am J Ophthalmol 2017;177:182-94.
20. Becker MD, Heiligenhaus A, Hudde T, et al. Interferon as a treatment for uveitis associated with multiple sclerosis. Br J Ophthalmol 2005;89:10:1254-7.
21. Qian Z, Fardeau C, Cardoso JN, et al. Effect of interferon α2a in cystoid macular edema due to intraocular infection. Eur J Ophthalmol 2015;25:5:431-6.
22. Oray M, Onal S, Uludag G, et al. Interferon alpha for the treatment of cystoid macular edema associated with presumed ocular tuberculosis. J Ocul Pharmacol Ther 2017;33:4:304-12.
23. Fattovich G, Giustina G, Favarato S, et al. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol 1996;24:1:38-47.
24. Okanoue T, Sakamoto S, Itoh Y, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283-91.
25. Muselier A, Bielefeld P, Bidot S, et al. Efficacy of tocilizumab in two patients with anti-TNF-alpha refractory uveitis. Ocul Immunol Inflamm 2011;19:5:382-3.
26. van Kooij B, Rothova A, Rijkers GT, et al. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. Am J Ophthalmol 2006;142:1:192-4.
27. Mesquida M, Molins B, Llorenç V, et al. Targeting interleukin-6 in autoimmune uveitis. Autoimmun Rev. 2017 Aug 2. (Epub ahead of print)
28. Zahir-Jouzdani F, Atyabi F, Mojtabavi N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology. 2017 Jun 10. (Epub ahead of print)
29. Adán A, Llorenç V, Mesquida M, et al. Tocilizumab treatment for recalcitrant uveitic macular edema. Graefes Arch Clin Exp Ophthalmol 2013;251:9:2249-50.
30. Adán A, Mesquida M, Llorenç V, et al. Tocilizumab treatment for refractory uveitis-related cystoid macular edema. Graefes Arch Clin Exp Ophthalmol 2013;251:11:2627-32.
31. Mesquida M, Molins B, Llorenç V, et al. Long-term effects of tocilizumab therapy for refractory uveitis-related macular edema. Ophthalmology 2014;121:12:2380-6.
32. Mesquida M, Molins B, Llorenç V, et al. Twenty-four month follow-up of tocilizumab therapy for refractory uveitis-related macular edema. Retina. 2017 May 16. (Epub ahead of print)
33. Tappeiner C, Mesquida M, Adán A, et al. Evidence for tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol 2016;43:12:2183-8.
34. Calvo-Río V, Santos-Gómez M, Calvo I, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis-associated uveitis refractory to anti-tumor necrosis factor therapy: A multicenter study of twenty-five patients. Arthritis Rheumatol 2017;69:3:668-75.
35. Calvo-Río V, Blanco R, Santos-Gómez M, et al. Efficacy of anti-IL6-receptor tocilizumab in refractory cystoid macular edema of birdshot retinochoroidopathy. Report of two cases and literature review. Ocul Immunol Inflamm 2016;11:1-6.
36. Deuter CME, Zierhut M, Igney-Oertel A, et al. Tocilizumab in uveitic macular edema refractory to previous immunomodulatory treatment. Ocul Immunol Inflamm 2017;25:2:215-20.
37. Kuijpers RW, Baarsma S, van Hagen PM. Treatment of cystoid macular edema with octreotide. N Engl J Med 1998;338:9:624-6.
38. Papadaki T, Zacharopoulos I, Iaccheri B, et al. Somatostatin for uveitic cystoid macular edema (CME). Ocul Immunol Inflamm 2005;13:6:469-70.
39. Kafkala C, Choi JY, Choopong P, et al. Octreotide as a treatment for uveitic cystoid macular edema. Arch Ophthalmol 2006;124:9:1353-5.
40. Missotten T, van Laar JA, van der Loos TL, et al. Octreotide long-acting repeatable for the treatment of chronic macular edema in uveitis. Am J Ophthalmol 2007;144:6:838-43.
41. Hussain MA, Studer K, Messmer EP, et al. Treatment with insulin-like growth factor I alters capillary permeability in skin and retina. Diabetes 1995;44:10:1209-12.
42. Fonollosa A, Coronado E, Catalan R, et al. Vitreous levels of somatostatin in patients with chronic uveitic macular oedema. Eye (Lond) 2012;26:10:1378-83.
43. Klok AM, Luyendijk L, Zaal MJ, et al. Soluble ICAM-1 serum levels in patients with intermediate uveitis. Br J Ophthalmol 1999;83:7:847-51.
44. Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol 2008;295:3:H969-77.
45. Wang J, Ibrahim M, Turkcuoglu P, et al. Intercellular adhesion molecule inhibitors as potential therapy for refractory uveitic macular edema. Ocul Immunol Inflamm 2010;18:5:395-8.
46. Faia LJ, Sen HN, Li Z, et al. Treatment of inflammatory macular edema with humanized anti-CD11a antibody therapy. Invest Ophthalmol Vis Sci 2011;52:9:6919-24.