JOAG: What We Know
Juvenile open-angle glaucoma is considered a subset of primary open-angle glaucoma, affecting about 1 in 50,000 persons (males and females equally). The definition has been controversial but generally takes into account the age of onset. The European Glaucoma Society defines JOAG as open-angle glaucoma with onset between the ages of 10 and 35 years.
JOAG demonstrates an autosomal dominant inheritance pattern, and research has found associations between JOAG and gene mutations on chromosome 1-1q21-q23. This locus is known as GLC1A, and contains the gene TIGR (trabecular meshwork induced glucocorticoid response) or MYOC,1,2 which codes for the myocilin protein. The exact function of this protein and its involvement in glaucoma is currently unknown. Forty mutations of the TIGR/MYOC gene have been identified in both juvenile and adult open-angle glaucoma, and genetic analyses have revealed that 8 to 63 percent of JOAG patients have a TIGR/MYOC mutation. Common findings among JOAG patients include early onset of disease, very high IOP and a strong family history of glaucoma.3,4 Histopathologic study of the trabecular meshwork in JOAG patients by Kyushu University’s Akihito Tawara, MD, and Hajime Inomata, MD, revealed an abnormally compact trabecular meshwork with an accumulation of extracellular material in the trabecular spaces.5
Clinical Findings
Juvenile open-angle glaucoma is generally asymptomatic in its early stages. Unlike primary infantile glaucoma, signs such as enlargement of the cornea and globe, breaks in Descemet’s membrane, corneal edema, epiphora and photophobia aren’t present in patients with the onset of open-angle glaucoma in later childhood or adolescence. Symptoms are rare but may include blurred vision and ocular pain from elevated intraocular pressure. Visual loss accompanies the later stages of the disease and often leads to patients seeking ophthalmic evaluation. Axial myopia has been associated with JOAG. Clinical signs include severe IOP elevation, often in the range of 40 to 50 mmHg. Given the lack of early symptoms, presentation is often late, and advanced cupping of the optic nerve is often noted on initial evaluation. Gonioscopic features include an open anterior chamber angle with high iris insertion and prominent iris processes.1 Normal-appearing optic discs and angles do not, however, rule out the diagnosis of JOAG.
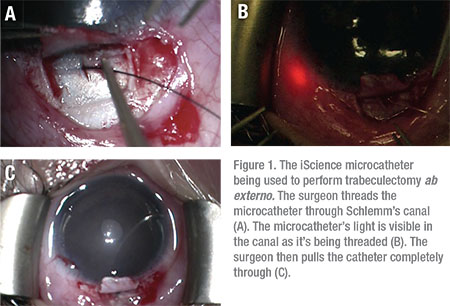 |
Exclude secondary causes of open-angle glaucoma when evaluating young patients with suspected JOAG. Note that pigment dispersion syndrome, uveitis, ocular trauma and steroid use can all result in elevated IOP and glaucoma, and a good clinical exam and review of systems is important to rule out any evidence of these conditions.
Treatment
Although up to 83 percent of JOAG patients eventually require surgical intervention,7 medical therapy may act as a bridge to more definitive surgical treatment. First-line topical therapy includes beta blockers, prostaglandin analogs and carbonic anhydrase inhibitors. Use alpha agonists with caution, or avoid them altogether, in young patients with JOAG, as potential adverse reactions have been reported in infants and toddlers, including bradycardia, hypotension, hypothermia, hypotonia, apnea and lethargy.8
When medications fail to control the IOP, the physician has to turn to surgery. Here’s a look at your options:
• Angle procedures. Angle-based surgical procedures are generally performed first, and are often effective in reducing the IOP and minimizing potential short- and long-term complications. The choice between goniotomy and trabeculotomy depends upon surgeon preference and experience.
Trabeculotomy, via an ab externo approach, has been reported to have a success rate of up to 86 percent in treating JOAG, with 14 percent of patients requiring additional surgical intervention to control IOP.9 Trabeculotomy ab externo may be used to incise the trabecular meshwork over 360 degrees using a suture or the iScience microcatheter. (See Figure 1)
Newer surgical techniques, such as gonioscopy-assisted transluminal trabeculotomy, have been developed and found to be useful in this patient population, as well. When performing the GATT procedure, the surgeon makes a small, initial 1-to-2 mm nasal goniotomy, and advances a microcatheter circumferentially through Schlemm’s canal. The catheter is pulled through to create a 360-degree cleft. The major benefit of ab interno procedures such as goniotomy, GATT and Trabectome is that they’re performed entirely through a corneal incision, avoiding conjunctival and scleral incisions—and subsequent scarring—altogether.
In cases where angle surgery fails to control IOP, further surgical options include external drainage procedures such as trabeculectomy with mitomycin-C and glaucoma drainage implant surgery, as well as cyclodestructive procedures.
• Trabeculectomy. Researchers have reported success rates with trabeculectomy ranging from 50 to 87 percent in JOAG patients.11-14 Obtaining and then maintaining a well-functioning filtering bleb in a child can be difficult, as younger patients have a more robust healing response, often resulting in progressive subconjunctival and episcleral fibrosis. Postop management, including adherence to eye-drop regimens and manipulations such as laser lysis of flap sutures, is complicated in very young children due to their inability to fully cooperate.
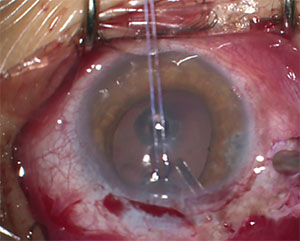 |
| Figure 2. This 13-year-old with JOAG required the placement of a glaucoma drainage device to control his IOP. |
Some clinicians have used antifibrosis therapy with MMC to reduce the amount of scarring in young patients, and this approach can result in lower IOPs following trabeculectomy in JOAG patients.11 However, the use of MMC has also been associated with an increased risk of vision-threatening complications, including hypotony maculopathy and bleb-related infection. The rate of hypotony maculopathy has been reported to be as high as 20 percent, which may be due to the increased incidence of axial myopia in these patients.11 One study found a 17-percent incidence of bleb-related infections in children with functional blebs.12 Given these findings, use caution when performing trabeculectomy with MMC in younger children.
• Glaucoma drainage implants. Due to the higher likelihood of conjunctival scarring in JOAG patients and the increased risk of sight-threatening complications with trabeculectomy (in younger patients), GDI surgery is a reasonable alternative. The most commonly used GDIs are the Ahmed glaucoma valve (New World Medical, Rancho Cucamonga, Calif.) and Baerveldt glaucoma implant (Abbott Medical Optics, Santa Ana, Calif.). Prior studies with at least one year of follow-up have documented success rates following pediatric GDI surgery ranging from 31 percent to 97 percent.15 There is, however, limited long-term data regarding GDI use in children and young adults.
Another potential issue with GDI in children is that proper positioning of the tube in the anterior chamber can be particularly challenging. Reduced scleral rigidity in these patients makes anterior migration and rotation of the proximal tube tip more common than in adults.16 Tube migration can result in direct contact or proximity of the tube tip to the posterior corneal surface and may contribute to endothelial cell loss and eventual corneal decompensation. Tube positioning as shown in Figure 2 is preferred; note the longer length and how it’s angled away from the corneal endothelium.
Given that patients with JOAG are young and often have very elevated IOPs with advanced disc damage, our preference is to use a 350-mm2 Baerveldt implant. We use a 7-0 polyglactin suture to temporarily ligate the tube of this non-valved device and allow three to four weeks for a capsule to form around the scleral explant.
For young children, we perform planned ligature release in the operating room between postoperative weeks three and four using a Hoskins lens and green diode laser.17 We make visualization of the ligature for laser lysis easier with the use of corneal tissue as the patch graft material. Fluid elevation of the conjunctiva overlying the scleral plate and profound softening of the globe help confirm ligature release. If you’re uncertain if the ligature has been released, you can use B-mode echography to demonstrate the presence of fluid around the drainage plate.
To replace lost volume and provide increased resistance to flow through the tube, you can inject sodium hyaluronate (10 mg/ml) into the anterior chamber immediately following ligature release. This limits the duration and magnitude of hypotony and prevents anterior chamber collapse. For younger children, we often will let the ligature release spontaneously, but will monitor them more closely after the first few postoperative weeks. For older, cooperative children and adults, you can perform planned ligature release by laser lysis followed by an anterior chamber injection of sodium hyaluronate (10 mg/ml) in the office. We prefer to maintain patients on atropine 1% and reduce the number of aqueous suppressant medications whenever possible after the third postoperative week in anticipation of the tube opening. These techniques can help prevent prolonged periods of hypotony and its secondary complications following ligature release.
Although GDI surgery and trabeculectomy have evolved over the years with advances in surgical technique and better ways to modulate wound healing, unique challenges exist in young patients with JOAG as compared to older adults. Careful preop assessment and postoperative monitoring, with frequent follow-up and attention to the early detection and management of adverse events, can improve long-term outcomes. REVIEW
Dr. Menezes is a resident physician at New York Eye and Ear Infirmary of Mt Sinai. Dr. Panarelli is an assistant professor of ophthalmology and associate residency program director at the New York Eye and Ear Infirmary of Mt Sinai. They have no financial interest in any of the products mentioned.
1. Albert DM, Miller JW, Azar DT, Blodi BA, Cohan JE, Perkins T. Juvenile Open Angle Glaucoma. In: Albert & Jakobiec’s Principles & Practice of Ophthalmology. 3rd ed. Philadelphia: Elsevier 2008.
2. Bruttini M, Longo I, Frezzotti P, et al. Mutations in the myocilin gene in families with primary open-angle glaucoma and juvenile open-angle glaucoma. Arch Ophthalmol 2003;121:7:1034-8.
3. Wiggs JL, Allingham RR, Vollrath D, et al. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet 1998;63:5:1549-52.
4. Adam MF, Belmouden A, Binisti P, et al. Recurrent mutation in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet 1997;6:12:2091-7.
5. Tawara A, Inomata H. Developmental immaturity of the trabecular meshwork in juvenile glaucoma. Am J Ophthalmol 1984;15:98:82-97.
6. Ko YC, Liu CJ, Chou JC, et al. Comparisons of risk factors and visual field changes between juvenile-onset and late-onset primary open angle glaucoma. Ophthalmologica 2002;216:1:27.
7. Wiggs JL, DelBono EA, Schuman JS, et al. Clinical features of five pedigrees genetically linked to the juvenile glaucoma locus on chromosome 1q21-q31. Ophthalmology 1995;102:1782-9.
8. Carlsen JO, Zabriskie NA, Kwon YH, et al. Apparent central nervous system depression in infants after the use of topical brimonidine. Am J Ophthalmol 1999;128:255-256.
9. Ikeda H, Ishigooka H, Muto T, et al. Long-term outcome of trabeculotomy for the treatment of developmental glaucoma. Arch Ophthalmol 2004;122:1122-8.
10. Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: An ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol 2015;99:8:1092-6.
11. Tsai JC, Chang HW, Kao CN, et al. Trabeculectomy with mitomycin C versus trabeculectomy alone for juvenile primary open-angle glaucoma. Ophthalmologica 2003;217:1:24-30.
12. Sidoti PA, Belmonte SJ, Liebmann JM, Ritch R. Trabeculectomy with mitomycin-c in the treatment of pediatric glaucomas. Ophthalmology 2000;107:422-9
13. Groh MJ, Behrens A, Handel A, Kuchle M. Mid- and long-term results after trabeculectomy in patients with juvenile and late-juvenile open-angle glaucoma. Klin Monatsbl Augenheilkd 2000;217:71-76
14. Aponte EP, Diehl N, Mohney BG. Medical and surgical outcomes in childhood glaucoma: A population-based study. J AAPOS 2011;15:3:263-7.
15. Chen TC, Chen PP, Francis BA, et al. Pediatric glaucoma surgery: A report by the AAO. Ophthalmology 2014:121:11:2107.
16. Netland PA. Walton DS. Glaucoma drainage implants in pediatric patients. Ophthalmic Surg 1993;24:723-729.
17. Sidoti PA, Panarelli, JF, Huruta-Dias R, Jardim J, Leon-Rosen J, Rosen RB. Laser tube ligature release following aqueous shunt implantation in young children. Ophthalmic Surg Lasers Imaging 2011;42:2:168-9.



