 |
Avoiding Complications
After performing approximately 800 endothelial keratoplasty procedures of all types since 2003, I have incorporated a number of key techniques to reduce the risk of complications during and after. Following are important preoperative and postoperative factors that the surgeon should consider before performing EK.
• Graft diameter sizing. The diameter of the Descemet’s stripping automated endothelial keratoplasty, or DSAEK, graft can be an important factor in long-term outcome. However, there is a controversy regarding DSAEK graft diameter sizing, with some experienced surgeons advocating the use of 9.5-mm grafts on everyone. In contrast, the diameter that I use most frequently is 8 mm. This particular size provides ample endothelial cells for long-term graft survival and allows for an easier insertion, with decreased compression of the tissue. An 8-mm diameter graft is situated far enough away from the anterior chamber angle and the paracentesis incisions, which potentially decreases the chance for peripheral anterior synechiae formation and allows the surgeon easy access to the anterior chamber for air-fluid exchange at the end of the case.
In patients with large corneal diameters or an increased anterior chamber depth, an increase in diameter to 8.5 to 9.5 mm may be appropriate, however I rarely use graft diameters in this range. In special cases such as buphthalmia or high myopia, though, an increase in diameter to 9.5 mm is common in my practice. In general, smaller diameter DSAEK buttons are associated with fewer complications, because the insertion is easier, less air is needed for adhesion, and there is a decreased chance of PAS formation. For these reasons, I recommend that the beginning DSAEK surgeon use grafts that are 8 to 8.25 mm in diameter. Then, with experience, larger diameter buttons can be used more reliably. Keep in mind that current research at Devers Eye Institute in Portland, Ore., has clearly shown that DSAEK with a graft diameter of 8.5 mm does not offer a clinical advantage over smaller 8-mm grafts, with respect to postoperative endothelial cell counts at two years after surgery.1
At the present time, there is a trend toward the use of ultra-thin DSAEK tissue, which has a thickness of 50 to 75 µm, rather than the traditional 100 to 200 µm. Ultra-thin DSAEK donors probably do provide a faster recovery and allow the surgeon to use newer, small incision insertion devices, since thinner tissue is less likely to become damaged from compression. However, in terms of complications, ultra-thin grafts may have a higher risk of postoperative striae that could impair vision and are more difficult to handle during and after insertion (especially for the inexperienced DSAEK surgeon). It should be noted that microstriae after DSAEK often improve with time, although macrostriae usually will require disc replacement.
For patients who have undergone penetrating keratoplasty and require endothelial transplantation, I recommend the use of a DSAEK button that’s the same diameter as the previous PK. Thinner DSAEK grafts ranging from 80 to 100 µm may also provide a better chance of adherence and conformation to the irregular posterior corneal bed sometimes seen after PK.
• Insertion considerations. Although many different types of insertion techniques for EK have been used here at the University of Iowa, the majority of our cases are still performed using a bifold forceps delivery technique with Charlie II insertion forceps (Bausch + Lomb/Storz Ophthalmics). Bifold forceps insertion using a 40/60 folding technique as described by Mark Terry, MD,2 is straightforward and is a technique that the average ophthalmology resident can master. On occasion, Busin glide (Moria) or cartridge injector (Monarch A, Alcon Surgical) suture pull-through techniques are used, however these instances are less frequent.
In order to prevent complications with any insertion technique, the key to is to protect the endothelium, which we do by coating the endothelium with a cohesive ophthalmic viscosurgical device. Sodium hyaluronate (Healon, Abbott Medical Optics) is preferred at our institution. During bifold forceps insertion, the incision size is important, because trauma to the endothelium may occur if the incision is too small. Because of this concern, a 5-mm incision is recommended in DSAEK. The same incision size is used with the Busin glide to prevent compression of the tissue and secondary loss of endothelium. With cartridge injector suture pull-through techniques, a 3.5- to 4-mm clear corneal incision may be used, and endothelial cell loss is similar to that seen with bifold forceps insertion in DSAEK.3
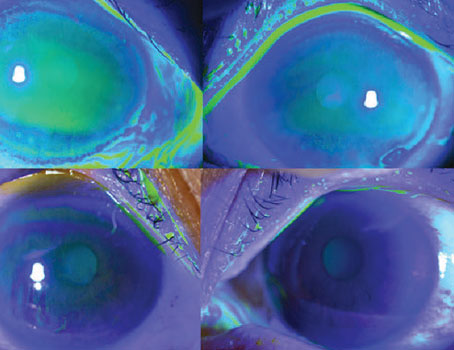
• Peripheral anterior synechiae. This complication is likely to occur after DSAEK if any of the incisions are incompetent and leak air or fluid. To avoid it, one has to standardize the size of the paracentesis ports. For instance, many surgeons, especially those new to DSAEK, may use a 15-degree sharp knife for their paracentesis incision creation. However, it may be difficult to standardize incision size with such blades. For that reason, I recommend using a paracentesis knife with a pre-determined width ranging from 0.9 to 1.5 mm, which ensures that the incision is the same size with every case. Depending on the size of the blade, the surgeon should know whether a suture will be necessary to close the wound to prevent loss of aqueous or air.
The paracentesis incisions should be made far enough in the periphery and away from the edges of the DSAEK button. This helps ensure that the disc does not dislocate during the air/fluid exchange or from the collection of fluid in the interface.
• Stripping the recipient. There is a debate in keratoplasty circles about the merit of stripping Descemet’s membrane from the recipient bed before insertion of the donor disc, especially in DSAEK after previous PK. One group of surgeons feels that stripping DM is an unnecessary step and that doing so risks dehiscence of the keratoplasty wound. In contrast, there is another group of surgeons, myself included, who strip DM in DSAEK after previous PK. I believe that stripping DM provides better postoperative vision. It can be done safely without the creation of PK wound dehiscence. In the setting of PK wound dehiscence during DSAEK, suture closure is imperative, and may affect disc adherence. Beginning surgeons should consider that the presence of high post-keratoplasty astigmatism may be a sign of wound instability and, in this setting, repeat PK rather than DSAEK may be the preferred procedure. In a multicenter retrospective study of DSAEK after PK reported by John Clements, MD, of the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., there was no statistically significant difference in DSAEK graft adherence depending on whether DM was stripped or left intact.4
• Roughen the stroma. I am in the ideological camp that advocates roughening the recipient stromal surface prior to placing the DSAEK donor graft. However, I make sure to just roughen the peripheral cornea, outside the visual axis. I feel that this step improves adhesion of the graft and helps avoid postoperative DSAEK dislocations. Though there are many instruments that can be used for this, I currently use the Melles DM scraper (DORC). It is true that some surgeons feel that this is an unnecessary step, but I believe that it makes a difference. It is logical that a rougher stromal surface would improve adhesion and reduce the DSAEK detachment rate.
After inserting the DSAEK donor button into the recipient, it is important to elevate the intraocular pressure to around 30 to 40 mmHg, to really lock the disc into position. The IOP should remain at that level for approximately five to seven minutes. After that period of high IOP, the pressure can be slightly reduced to the 20- to 30-mmHg range, but the surgeon has to ensure that there is no interface fluid or a collection of fluid around the edges of the disc. I usually sit in the operating room with the patient for approximately 15 minutes to make sure the disc has adhered properly. One area where surgeons can possibly get into trouble intraoperatively is during the exchange of air for balanced salt solution. It’s important to make sure that there’s no air behind the iris prior to leaving the operating room.
• Peripheral anterior synechiae. This complication is likely to occur after DSAEK if any of the incisions are incompetent and leak air or fluid. To avoid it, one has to standardize the size of the paracentesis ports. For instance, many surgeons, especially those new to DSAEK, may use a 15-degree sharp knife for their paracentesis incision creation. However, it may be difficult to standardize incision size with such blades. For that reason, I recommend using a paracentesis knife with a pre-determined width ranging from 0.9 to 1.5 mm, which ensures that the incision is the same size with every case. Depending on the size of the blade, the surgeon should know whether a suture will be necessary to close the wound to prevent loss of aqueous or air.
The paracentesis incisions should be made far enough in the periphery and away from the edges of the DSAEK button. This helps ensure that the disc does not dislocate during the air/fluid exchange or from the collection of fluid in the interface.
• Stripping the recipient. There is a debate in keratoplasty circles about the merit of stripping Descemet’s membrane from the recipient bed before insertion of the donor disc, especially in DSAEK after previous PK. One group of surgeons feels that stripping DM is an unnecessary step and that doing so risks dehiscence of the keratoplasty wound. In contrast, there is another group of surgeons, myself included, who strip DM in DSAEK after previous PK. I believe that stripping DM provides better postoperative vision. It can be done safely without the creation of PK wound dehiscence. In the setting of PK wound dehiscence during DSAEK, suture closure is imperative, and may affect disc adherence. Beginning surgeons should consider that the presence of high post-keratoplasty astigmatism may be a sign of wound instability and, in this setting, repeat PK rather than DSAEK may be the preferred procedure. In a multicenter retrospective study of DSAEK after PK reported by John Clements, MD, of the Edward Hines Jr. Veterans Affairs Hospital in Hines, Ill., there was no statistically significant difference in DSAEK graft adherence depending on whether DM was stripped or left intact.4
• Roughen the stroma. I am in the ideological camp that advocates roughening the recipient stromal surface prior to placing the DSAEK donor graft. However, I make sure to just roughen the peripheral cornea, outside the visual axis. I feel that this step improves adhesion of the graft and helps avoid postoperative DSAEK dislocations. Though there are many instruments that can be used for this, I currently use the Melles DM scraper (DORC). It is true that some surgeons feel that this is an unnecessary step, but I believe that it makes a difference. It is logical that a rougher stromal surface would improve adhesion and reduce the DSAEK detachment rate.
After inserting the DSAEK donor button into the recipient, it is important to elevate the intraocular pressure to around 30 to 40 mmHg, to really lock the disc into position. The IOP should remain at that level for approximately five to seven minutes. After that period of high IOP, the pressure can be slightly reduced to the 20- to 30-mmHg range, but the surgeon has to ensure that there is no interface fluid or a collection of fluid around the edges of the disc. I usually sit in the operating room with the patient for approximately 15 minutes to make sure the disc has adhered properly. One area where surgeons can possibly get into trouble intraoperatively is during the exchange of air for balanced salt solution. It’s important to make sure that there’s no air behind the iris prior to leaving the operating room.
After the air/BSS exchange, air is re-injected into the anterior chamber so that a mobile air bubble that covers the edges of the disc is present. If the pressure at that point is low, perhaps less than normal IOP (less than 15 mmHg), the risk of graft non-adherence increases. Also, the paracentesis incisions should be closely examined. If the security of the incisions’ closure is unclear, frank leakage of air or aqueous is present, or if maintenance of the anterior chamber is difficult, the incisions must be closed with a nylon suture.
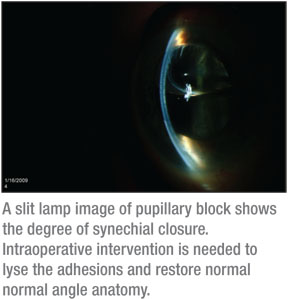
• Avoiding pupillary block. For the beginning surgeon, I recommend following the techniques used at the Devers Eye Institute to help avoid pupillary block.2 Although I use the same technique for DSAEK, on occasion I’ve observed that there are certain patients who require more air to get their discs to adhere. And, if you as the surgeon push the clinical envelope by adding more air into the anterior chamber, the risk of pupillary block markedly increases. Because of this risk, I think the placement of an inferior peripheral iridectomy is invaluable. As part of standard DSAEK at our institution, an inferior peripheral iridectomy is created manually with microscissors, either the Duet scissors (Microsurgical Technology) or 23-ga. scissors (Alcon Surgical).
Postoperatively, bleeding could be a problem after creation of the iridectomy, leading to blockage and secondary pupillary block, although this has been rare in my experience. More often than not, the inferior iridectomy is successful and improves my outcomes, especially in patients with poor pupillary dilation and/or floppy iris syndrome.
• Eliminate fluid in the interface. I advocate post-DSAEK disc insertion corneal massage as a way to improve the chance of adherence. For this, we use either the Bausch + Lomb/Storz Ophthalmics’ Bishop-Harmon irrigating cannula or the company’s Cindy Sweeper for DSEK to glide back and forth across the cornea to force fluid out of the graft interface.
I am not a proponent of trying to evacuate the interface fluid with corneal venting incisions. I believe these incisions increase the risk of lamellar interface infection and epithelial ingrowth. On the other hand, if the patient is status-post PK and is now undergoing DSAEK with a visible gap between the graft and the host bed, the placement of a venting incision with a diamond micrometer knife is appropriate.
Managing Complications
Even the best technique can result in complications after DSAEK surgery. Here’s how you can respond if they occur.
• Patient factors. Certain patient attributes will increase the risk of complications, regardless of how well the surgery is done. For example, a patient with dementia is more likely to rub the eye and induce a graft detachment. Glaucoma patients with a functioning seton or trabeculectomy filtration site are more likely to experience hypotony after surgery, and concomitant graft dislocation. African-American patients are three to five times more likely to have an allograft rejection episode after PK or EK.5-6
To help prevent allograft rejection after DSAEK, a higher than normal administration of topical corticosteroids may be needed. The standard postoperative corticosteroid regimen after keratoplasty at the University of Iowa is q.i.d. for three months, t.i.d. for three months, b.i.d. for three months and q.d. thereafter. However, for patients at higher risk for allograft rejection, topical cyclosporine 0.05% to 2% drops b.i.d may be useful. Topical application of cyclosporine may help improve the ocular surface, prevent allograft rejection and decrease steroid administration in patients with secondary glaucoma.
• Graft dislocation. Decentration of the graft, which may be induced by eye rubbing and/or postoperative hypotony, doesn’t always require surgical intervention. For instance, if a patient presents a week to 10 days after surgery with a DSAEK disc that has shifted position, but remains primarily in the visual axis, observation is recommended. This is because, once the disc has adhered, trying to detach and reposition again increases the risk of damaging the endothelium. In most cases, these patients function very well, with excellent vision.
However, if the disc is dislocated so that its edge is in the visual axis and creating a reduction in best-corrected vision, there is no other choice except to reposition or replace the DSAEK tissue, depending on surgeon skill and patient factors.
When approaching a repositioning procedure, it should be done as if it were a primary case with standard sterility precautions. I have access to a minor procedure room in our office that is dedicated to keratoplasty repositioning and re-suturing. The patient receives a Betadine 5% scrub and fornix irrigation, and is then prepped and draped. The anxious patient receives oral diazepam 5 mg and intracameral lidocaine 1% as needed, however most patients function well with topical tetracaine alone.
Using BSS to maintain the anterior chamber, the DSAEK tissue can be repositioned through one of the paracentesis incisions using a small air bubble. After centration, all of the BSS is exchanged for air, and the IOP is increased to lock the disc into position, taking care that no fluid touches the edges of the disk. After 15 minutes, the bubble size is reduced to cover the edges of the disc, and the patient lies flat for another 60 to 90 minutes prior to leaving.
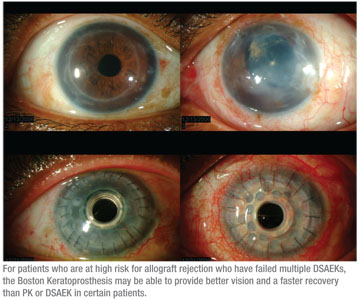
• When a graft fails. If a DSAEK graft fails early in the postoperative period, my response depends on the particular situation. If the patient is a “high-risk” keratoplasty subject, with concomitant glaucoma, seton placement or active trabeculectomy, other treatment alternatives must be considered. In our keratoplasty database at the University of Iowa, we have found that patients with significant glaucoma have better graft survival with a PK instead of DSAEK. Additional research suggests that the Boston Keratoprosthesis is also an excellent option.7
For patients who are at high risk for allograft rejection who have failed multiple DSAEK procedures, conversion to a KPro has proved to be successful at our center. The KPro may provide better vision and a faster recovery compared to PK or DSAEK in certain patients.
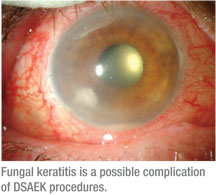
• Infection. There have been clinical reports of fungal infection deep in the interface after DSAEK,8 and bacterial contamination has recently been described, as well.9 Although the two types of infection may appear similar, the timing of the infection may be the best indication as to the specific cause. Bacterial infections usually develop rapidly, appearing within a few days of the procedure. In contrast, fungal contamination develops more insidiously over a period of weeks to months.
In both situations, if the endothelial graft and the recipient cornea show infiltration, a PK will be required to ensure that the infection is eradicated. If only the endothelial graft is affected, removal of the DSAEK button is the logical choice.
It’s important for the surgeon to culture the DSAEK donor rim for fungus at the time of surgery. If a positive fungal culture is treated prior to clinical presentation, there is a great chance that fungal keratitis in either the DSAEK button and/or recipient can be avoided.
• Cystoid macular edema. This complication is managed by our retina service, and the therapy will start with the use of a topical corticosteroid and non-steroidal anti-inflammatory drops in combination. This will be followed by sub-Tenon’s triamcinolone injection, intravitreal triamcinolone or implantation of a Retisert (fluocinolone sustained release implant), depending on the severity of disease.
• Suprachoroidal hemorrhage. Fortunately, this is rare after EK but, when it occurs, tight wound closure is a must. The wound closure is followed by oral corticosteroids to reduce inflammation. Surgical drainage of the suprachoroidal space after liquification occurs in 10 to 14 days is the mainstay of treatment. In my experience, suprachoroidal hemorrhage results in loss of the EK graft due to allograft rejection and glaucoma.
In closing, DSAEK is a great procedure that is actually easy to master. The excellent results and reproducibility of the procedure ensure that it will be a workhorse for the management of endothelial dysfunction for quite a while.
Dr. Goins is a professor of clinical ophthalmology, and director of the refractive/laser surgery, cornea and external disease service at the University of Iowa’s Department of Ophthalmology and Visual Sciences. He is also medical director of the Iowa Lions Eye Bank.
1. Terry MA, Li J, Goshe J, Davis-Boozer D. Endothelial keratoplasty: The relationship between donor tissue size and donor endothelial survival. Ophthalmology 2011 Jun6 [Epub ahead of print]
2. Terry MA, Shamie N, Chen ES, Hoar KL, Friend DJ. Endothelial keratoplasty a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology 2008 Jul;115:7:1179-86.
3. Wendel LJ, Goins KM, Sutphin JE, Wagoner MD. Comparison of bifold forceps and cartridge injector suture pull-through insertion techniques for Descemet stripping automated endothelial keratoplasty. Cornea 2011;30:3:273-6.
4. Clements JL, Bouchard CS, Lee WB, Dunn SP, Mannis MJ, Reidy JJ, John T, Hannush, SB, Goins KM, Wagoner MD, Adi MA, Rubenstein JB, Udell IJ, Babiuch AS. Retrospective review of graft dislocation rate associated with descemet stripping automated endothelial keratoplasty after primary failed penetrating keratoplasty. Cornea 2011;30:4:414-8.
5. Price MO, Thompson RW, Price FW. Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol 2003;121:1087-1092.
6. Price MO, Jordan CS, Moore G, Price FW. Graft rejection episodes after Descemet Stripping with endothelial keratoplasty, Part two: The statistical analysis of probability and risk factors. Br J Ophthalmol 2009:93:3:391-5.
7. Zerbe BL, Belin MW, Ciolino JB; Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006;113:10:1779.e1-7.
8. Kitzmann AS, Wagoner MD, Syed NA, Goins KM. Donor-related Candida keratitis after Descemet’s stripping automated endothelial keratoplasty. Cornea 2009;28:7:825-8.
9. Sharma N, Agarwal PC, Kumar CS, Mannan R, Titiyal JS. Microbial keratitis after Descemet Stripping Automated Endothelial Keratoplasty. Eye and Contact Lens 2011; in press.
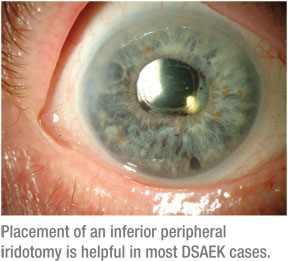 |
Postoperatively, bleeding could be a problem after creation of the iridectomy, leading to blockage and secondary pupillary block, although this has been rare in my experience. More often than not, the inferior iridectomy is successful and improves my outcomes, especially in patients with poor pupillary dilation and/or floppy iris syndrome.
• Eliminate fluid in the interface. I advocate post-DSAEK disc insertion corneal massage as a way to improve the chance of adherence. For this, we use either the Bausch + Lomb/Storz Ophthalmics’ Bishop-Harmon irrigating cannula or the company’s Cindy Sweeper for DSEK to glide back and forth across the cornea to force fluid out of the graft interface.
I am not a proponent of trying to evacuate the interface fluid with corneal venting incisions. I believe these incisions increase the risk of lamellar interface infection and epithelial ingrowth. On the other hand, if the patient is status-post PK and is now undergoing DSAEK with a visible gap between the graft and the host bed, the placement of a venting incision with a diamond micrometer knife is appropriate.
Managing Complications
Even the best technique can result in complications after DSAEK surgery. Here’s how you can respond if they occur.
• Patient factors. Certain patient attributes will increase the risk of complications, regardless of how well the surgery is done. For example, a patient with dementia is more likely to rub the eye and induce a graft detachment. Glaucoma patients with a functioning seton or trabeculectomy filtration site are more likely to experience hypotony after surgery, and concomitant graft dislocation. African-American patients are three to five times more likely to have an allograft rejection episode after PK or EK.5-6
To help prevent allograft rejection after DSAEK, a higher than normal administration of topical corticosteroids may be needed. The standard postoperative corticosteroid regimen after keratoplasty at the University of Iowa is q.i.d. for three months, t.i.d. for three months, b.i.d. for three months and q.d. thereafter. However, for patients at higher risk for allograft rejection, topical cyclosporine 0.05% to 2% drops b.i.d may be useful. Topical application of cyclosporine may help improve the ocular surface, prevent allograft rejection and decrease steroid administration in patients with secondary glaucoma.
• Graft dislocation. Decentration of the graft, which may be induced by eye rubbing and/or postoperative hypotony, doesn’t always require surgical intervention. For instance, if a patient presents a week to 10 days after surgery with a DSAEK disc that has shifted position, but remains primarily in the visual axis, observation is recommended. This is because, once the disc has adhered, trying to detach and reposition again increases the risk of damaging the endothelium. In most cases, these patients function very well, with excellent vision.
However, if the disc is dislocated so that its edge is in the visual axis and creating a reduction in best-corrected vision, there is no other choice except to reposition or replace the DSAEK tissue, depending on surgeon skill and patient factors.
When approaching a repositioning procedure, it should be done as if it were a primary case with standard sterility precautions. I have access to a minor procedure room in our office that is dedicated to keratoplasty repositioning and re-suturing. The patient receives a Betadine 5% scrub and fornix irrigation, and is then prepped and draped. The anxious patient receives oral diazepam 5 mg and intracameral lidocaine 1% as needed, however most patients function well with topical tetracaine alone.
 |
Using BSS to maintain the anterior chamber, the DSAEK tissue can be repositioned through one of the paracentesis incisions using a small air bubble. After centration, all of the BSS is exchanged for air, and the IOP is increased to lock the disc into position, taking care that no fluid touches the edges of the disk. After 15 minutes, the bubble size is reduced to cover the edges of the disc, and the patient lies flat for another 60 to 90 minutes prior to leaving.
• When a graft fails. If a DSAEK graft fails early in the postoperative period, my response depends on the particular situation. If the patient is a “high-risk” keratoplasty subject, with concomitant glaucoma, seton placement or active trabeculectomy, other treatment alternatives must be considered. In our keratoplasty database at the University of Iowa, we have found that patients with significant glaucoma have better graft survival with a PK instead of DSAEK. Additional research suggests that the Boston Keratoprosthesis is also an excellent option.7
For patients who are at high risk for allograft rejection who have failed multiple DSAEK procedures, conversion to a KPro has proved to be successful at our center. The KPro may provide better vision and a faster recovery compared to PK or DSAEK in certain patients.
 |
In both situations, if the endothelial graft and the recipient cornea show infiltration, a PK will be required to ensure that the infection is eradicated. If only the endothelial graft is affected, removal of the DSAEK button is the logical choice.
It’s important for the surgeon to culture the DSAEK donor rim for fungus at the time of surgery. If a positive fungal culture is treated prior to clinical presentation, there is a great chance that fungal keratitis in either the DSAEK button and/or recipient can be avoided.
• Cystoid macular edema. This complication is managed by our retina service, and the therapy will start with the use of a topical corticosteroid and non-steroidal anti-inflammatory drops in combination. This will be followed by sub-Tenon’s triamcinolone injection, intravitreal triamcinolone or implantation of a Retisert (fluocinolone sustained release implant), depending on the severity of disease.
• Suprachoroidal hemorrhage. Fortunately, this is rare after EK but, when it occurs, tight wound closure is a must. The wound closure is followed by oral corticosteroids to reduce inflammation. Surgical drainage of the suprachoroidal space after liquification occurs in 10 to 14 days is the mainstay of treatment. In my experience, suprachoroidal hemorrhage results in loss of the EK graft due to allograft rejection and glaucoma.
In closing, DSAEK is a great procedure that is actually easy to master. The excellent results and reproducibility of the procedure ensure that it will be a workhorse for the management of endothelial dysfunction for quite a while.
Dr. Goins is a professor of clinical ophthalmology, and director of the refractive/laser surgery, cornea and external disease service at the University of Iowa’s Department of Ophthalmology and Visual Sciences. He is also medical director of the Iowa Lions Eye Bank.
1. Terry MA, Li J, Goshe J, Davis-Boozer D. Endothelial keratoplasty: The relationship between donor tissue size and donor endothelial survival. Ophthalmology 2011 Jun6 [Epub ahead of print]
2. Terry MA, Shamie N, Chen ES, Hoar KL, Friend DJ. Endothelial keratoplasty a simplified technique to minimize graft dislocation, iatrogenic graft failure, and pupillary block. Ophthalmology 2008 Jul;115:7:1179-86.
3. Wendel LJ, Goins KM, Sutphin JE, Wagoner MD. Comparison of bifold forceps and cartridge injector suture pull-through insertion techniques for Descemet stripping automated endothelial keratoplasty. Cornea 2011;30:3:273-6.
4. Clements JL, Bouchard CS, Lee WB, Dunn SP, Mannis MJ, Reidy JJ, John T, Hannush, SB, Goins KM, Wagoner MD, Adi MA, Rubenstein JB, Udell IJ, Babiuch AS. Retrospective review of graft dislocation rate associated with descemet stripping automated endothelial keratoplasty after primary failed penetrating keratoplasty. Cornea 2011;30:4:414-8.
5. Price MO, Thompson RW, Price FW. Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol 2003;121:1087-1092.
6. Price MO, Jordan CS, Moore G, Price FW. Graft rejection episodes after Descemet Stripping with endothelial keratoplasty, Part two: The statistical analysis of probability and risk factors. Br J Ophthalmol 2009:93:3:391-5.
7. Zerbe BL, Belin MW, Ciolino JB; Boston Type 1 Keratoprosthesis Study Group. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006;113:10:1779.e1-7.
8. Kitzmann AS, Wagoner MD, Syed NA, Goins KM. Donor-related Candida keratitis after Descemet’s stripping automated endothelial keratoplasty. Cornea 2009;28:7:825-8.
9. Sharma N, Agarwal PC, Kumar CS, Mannan R, Titiyal JS. Microbial keratitis after Descemet Stripping Automated Endothelial Keratoplasty. Eye and Contact Lens 2011; in press.