While glaucoma is commonly associated with elevated intraocular pressure, a subset of primary open-angle glaucoma can be classified as normal-tension glaucoma, an optic neuropathy known to wreak the same ocular havoc as POAG. “Normal” refers to IOP measurements of 21 mmHg or lower. Terms like “low-tension” and “low-pressure” glaucoma have fallen out of favor because “low” is a relative term for this subset of patients, and current treatment strategy remains focused on lowering IOP. But the complexities in this subpopulation lay the groundwork for theories and research, which could have larger implications for future disease classification and treatment.
Here, experts discuss the current approaches to treatment and weigh in on alternative perspectives to simply characterizing patients as having normal tension glaucoma within a subset of POAG.
Unique Characteristics of NTG
Despite lower IOP and open anterior chamber angles seen on gonioscopy, NTG patients aren’t spared tell-tale optic nerve head damage, progressive retinal nerve fiber layer thinning and visual field defects.1 But there are nuances. “NTG patients tend to have more focal notching in the rim, and they’re more likely to develop disc hemorrhages or flame-shaped hemorrhages across the rim of the disc,” explains Leonard Seibold, MD, an associate professor of ophthalmology at the University of Colorado School of Medicine. “These often happen adjacent to preexisting notches in the rim. They also tend to have more paracentral scotomas on their visual field testing compared to high-pressure glaucomas, which typically affect peripheral vision early and central vision later.”
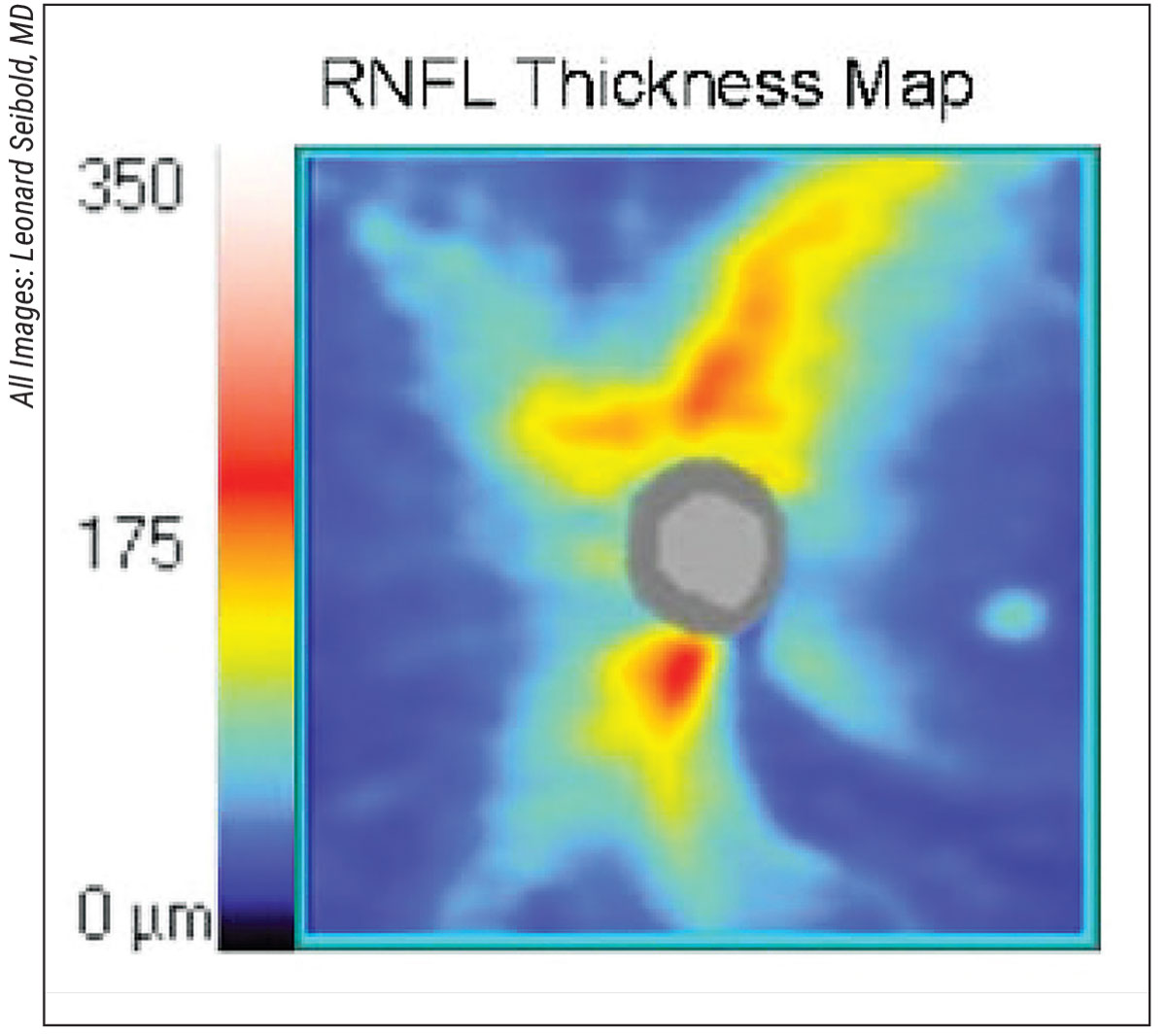 |
| Focal retinal nerve fiber layer thinning is seen in the inferotemporal location in an eye with normal-tension glaucoma. |
All Things Considered
“The diagnosis of normal-tension glaucoma is only reached after excluding other forms of optic neuropathy, such as ischemic, traumatic, toxic, inflammatory, infectious, congenital or compressive conditions,” said Razeghinejad Mohammadreza, MD, director of the Glaucoma Fellowship Program at Wills Eye Hospital in Philadelphia. “A neuroimaging evaluation is highly suggested in atypical cases, such as a younger patient with a decrease in visual acuity, impaired color vision, pallor of the neuroretinal rim, highly asymmetric cupping and VF defects respecting the vertical meridian.”
“It’s a diagnosis of exclusion,” agrees Peter A. Netland, MD, PhD, Vernah Scott Moyston professor and chair of ophthalmology at the University of Virginia School of Medicine in Charlottesville. He says that if the eyes are markedly asymmetrical and one eye is highly abnormal, for example, if it has a lot of cupping and optic nerve atrophy or other findings such as pallor of the neuroretinal rim or loss of color vision, and the patient is under [the age of] 60, then he considers a differential diagnosis. “That’s not to say a 40-year-old can’t have NTG,” says Dr. Netland, “but anything that’s not in the typical findings would raise my suspicion that it might be something else.”
NTG Masqueraders
Identifying NTG masqueraders is a challenge. “It could be some type of glaucoma the person may have had, which is now resolved or in remission,” or it could be a nonglaucomatous condition, notes Dr. Netland. “Classic examples include vascular diseases, [exposure to] certain toxins such as methanol, syphilis or congenital anomalies. There are many possibilities. We’re constantly looking for other diagnoses to make sure we can exclude them first.”
How Low Can You Go?
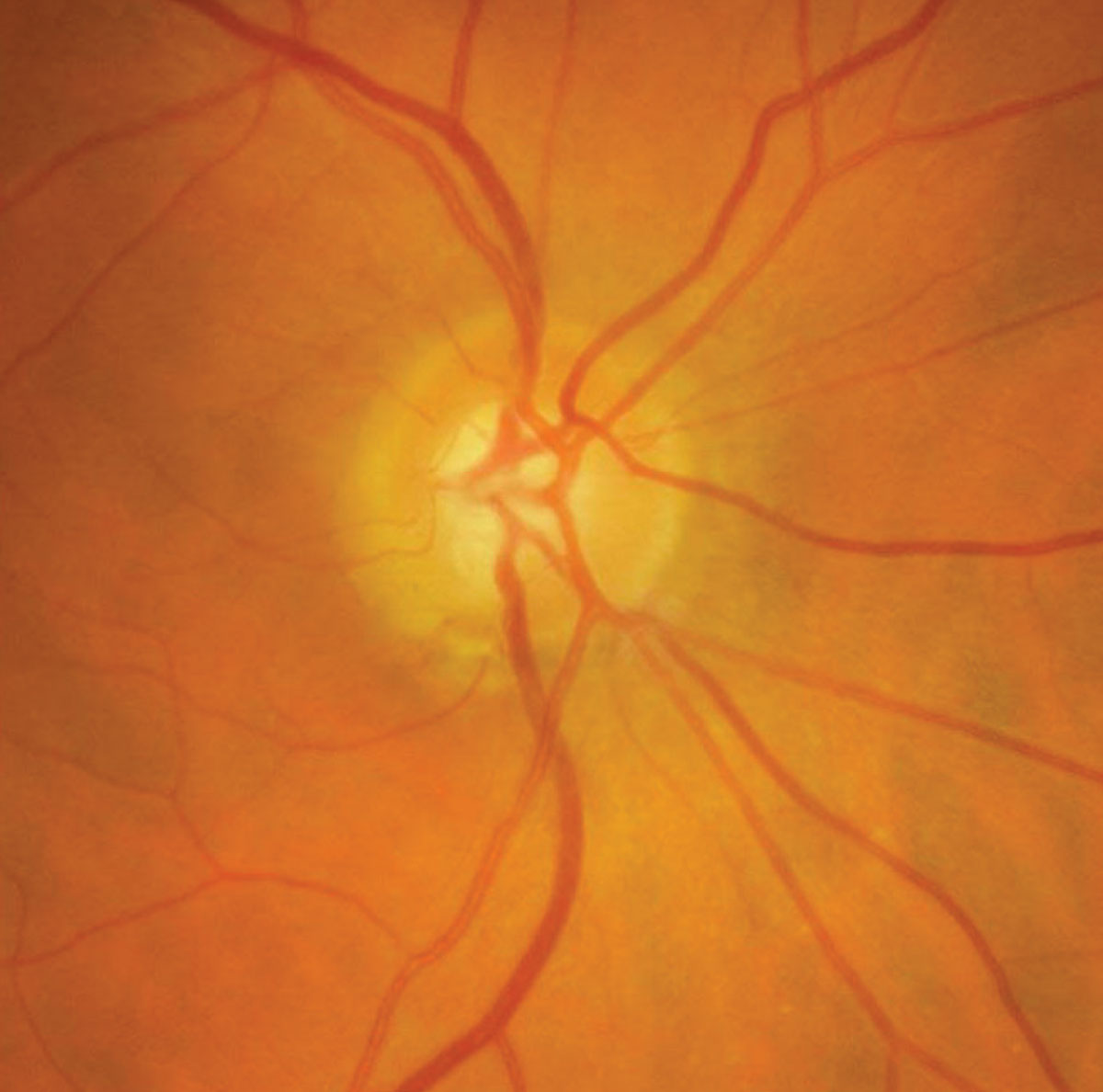 |
| Inferior optic disc rim notching in an eye with normal-tension glaucoma. |
For the most part, “we treat NTG the same as primary open-angle glaucoma,” explains Dr. Seibold. “We’re just starting from a normal pressure and getting to an even lower pressure.” He says this approach has become a standard since the 1998 Collaborative Normal Tension Glaucoma Treatment Study.2 “It proved that a further reduction in pressure does in fact alter the course of the disease, so we can stabilize a lot of disease by a 30-percent reduction in pressure,” Dr. Seibold says. “It’s a good guideline for most patients to lower pressure by at least 20 percent, but each eye is different. Some need a pressure of 12 mmHg, some need a pressure of 10 mmHg, and some need a pressure of 8 mmHg.”
Dr. Mohammadreza says that, as with POAG, “The treatment algorithm is the same and can include selective laser trabeculoplasty, medication and MIGS or filtering surgeries, based on the severity of the glaucoma.”
Medications and NTG
Prostaglandin analogs are the most widely prescribed glaucoma medications, but newer therapeutic classes for NTG nonresponders have broadened physicians’ options.
“Sometimes our treatment approach is a little nuanced, particularly for medications,” says Dr. Seibold. “Using rho kinase inhibitors can potentially lower episcleral venous pressure and may help us to get even lower pressures, playing more of a role in normal-tension glaucoma.”
Dr. Netland agrees the newer class of rho kinase inhibitors may have a role in NTG, noting that although they haven’t been studied in this population, their ability to increase the drainage of intraocular fluid could prove beneficial in lowering IOP for these patients.
He speculates that interest could resurge in drugs with neuroprotective or neurovascular effects that are used for lowering IOP, recalling the Low-Pressure Glaucoma Study Group from 2011. In that study, researchers compared brimonidine tartrate 0.2% to the beta-adrenergic antagonist timolol maleate 0.5%. The primary endpoint was preservation of visual function in low-pressure glaucoma, and statistically fewer brimonidine-treated patients (n=9; 9.1 percent) had visual field progression by pointwise linear regression than timolol-treated patients (n=31; 39.2 percent, log-rank 12.4, p=0.001). Mean IOP was similar in both groups.3 “I think it’s an interesting study and it has been influential,” Dr. Netland noted.
He says that carbonic anhydrase inhibitors “have known beneficial effects on retinal circulation,” but a magic bullet for NTG patients remains elusive.
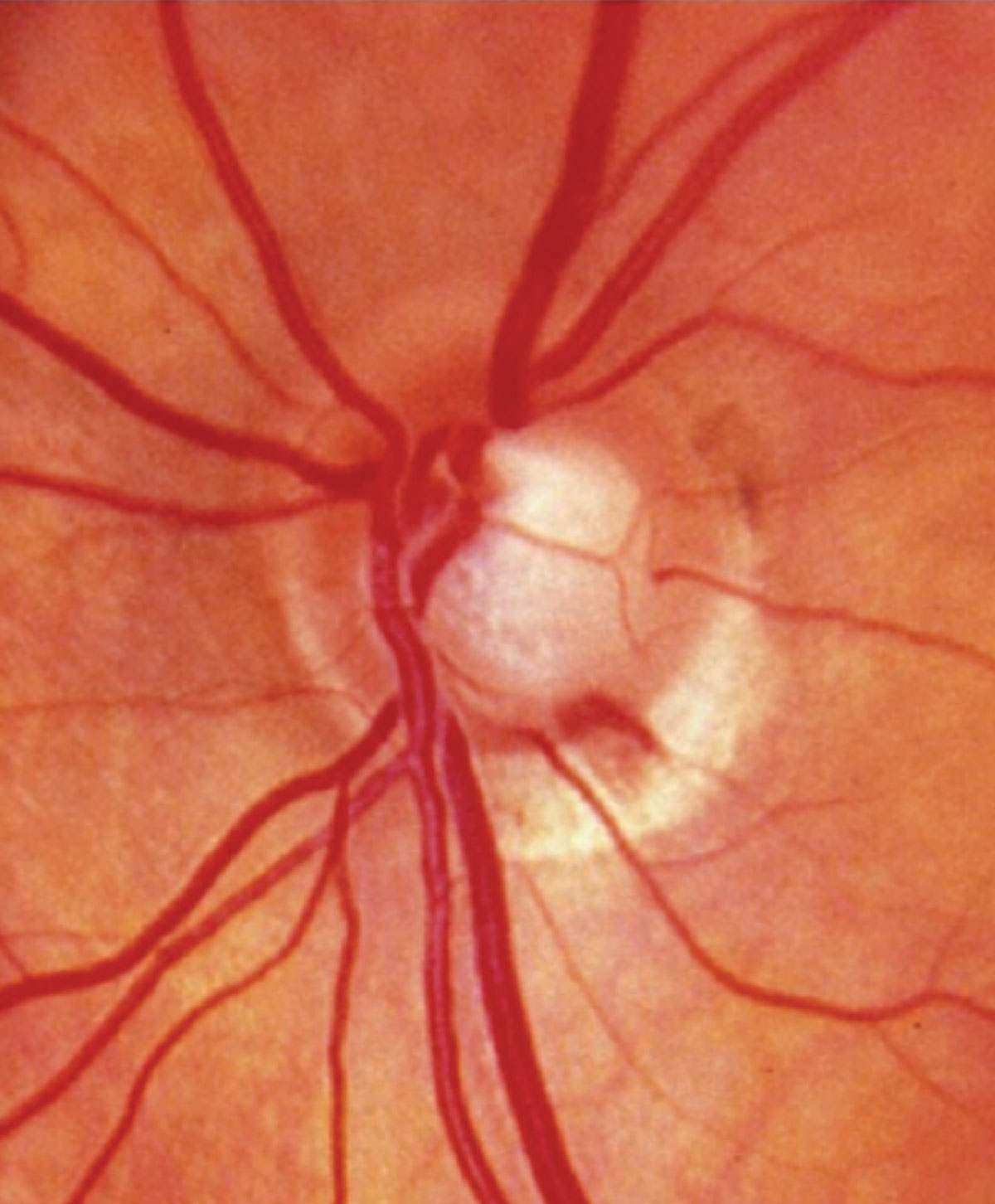 |
| Optic disc hemorrhage in an eye with normal-tension glaucoma. |
Lasers and Surgery
“Selective laser trabeculoplasty has been shown in NTG patients to reduce fluctuation of IOP, avoiding peaks in patients who are progressing,” notes Dr. Netland. “It’s used to keep average pressures in the mid- to low-teens. You may not see much effect on the average pressure you measure in the clinic, but the idea is that you might be cutting off undetected peaks. That’s been found to be the case in trials.
“Obviously all of us, including myself, are moving on to MIGS,” Dr. Netland continues, “particularly with transscleral devices and sometimes with adjunctive therapy. MIGS can achieve pressures that are close to or equivalent to SLT, so I think we’re shifting a bit more in that direction.”
However, Dr. Netland cautions that “some of the MIGS aren’t as effective in lowering IOP and may not achieve the goals we’re trying to target.”
Dr. Seibold agrees that MIGS has a role in NTG treatment, but acknowledges limitations. “A lot of the angle procedures like Kahook Dual Blade (New World Medical), iStent (Glaukos) and Hydrus (Ivantis) are very good at lowering pressure from the 20s and 30s back to a normal range, but when you’re starting in the mid-teens, they don’t offer a lot in the way of lowering IOP further, so we often have to go to a filtering surgery like a trabeculectomy to get those really low pressures under 12 mmHg,” he explains. “That’s why MIGS has less of a role in NTG.”
Patient Characteristics
Refining treatments to target a subgroup of patients is even more of a challenge when prevalence data comes into question. One paper cites a worldwide NTG prevalence range between 30 and 90 percent.4
And, while NTG is higher in Asian populations, “The median range of prevalence that’s been reported in the U.S. is about 20 to 30 percent of glaucoma patients,” notes Dr. Netland. “It’s certainly not rare. It’s not an uncommon condition.”
Some speculate that low daytime IOP measurements could misclassify NTG. “Some of these patients may have POAG with significant IOP fluctuation,” notes Dr. Mohammadreza. “Peak IOPs may be happening during out-of-office hours. Some of the NTG patients could be labeled as POAG after having a diurnal curve. A modified diurnal curve, which involves checking the IOP during office hours every one to two hours, certainly misses any IOP spikes happening at night, in the early evening, and in the morning.” Dr. Mohammadreza says that the iCare home tonometer could be useful for assessing diurnal variations.
Dr. Seibold agrees. “Studies show that up to two-thirds of patients will have their peak IOP outside of office hours,” he says. Thus, despite varying morning and afternoon visits to document IOP spikes, he says, “We may still miss it. That’s where home tonometry can now play a role, because we can have patients use a home tonometry device and measure those pressures after-hours: early morning; evening; late at night.”
Thus, “while NTG in the U.S. makes up about one-third to maybe as many as half of the glaucoma cases we see, it may be actually less than that, and it’s just misclassified because we’re missing the high pressures,” Dr. Seibold speculates.
Based on home tonometer readings, New York’s Icahn School of Medicine professor Louis R. Pasquale, MD, says, “We’re finding that pressures outside of the office are much higher than they are in the office.” This may explain why patients are progressing, but it also opens a can of worms for him in terms of NTG as a glaucoma category. Dr. Pasquale speculates that if more NTG patients checked IOP at off hours, they would see pressures higher than 21 mmHg. “Generally speaking, stratifying patients with glaucoma based on IOP has not been that informative,” he says. (See sidebar below, “Turning Normal-tension Glaucoma on its Head.")
Guilt by Association
Patients often want to know what causes their condition. Glaucoma specialists have few answers for those with NTG. “The etiology is unknown,” notes Dr. Netland. “A lot of things might influence NTG.” Associations include migraine and Raynaud’s syndrome, the latter of which suggests what Dr. Netland calls “some sort of vascular dysregulation.”
“Vascular dysregulation as a factor in the development of glaucoma seems to play a major role in NTG compared with POAG,” agrees Dr. Mohammadreza.
In addition, Dr. Netland says, “Various other problems have been associated with NTG, such as hypertension, hypotension, ocular perfusion pressure abnormalities, and there are some intriguing findings about CSF pressure.”
Turning Normal-tension Glaucoma on its Head But NTG is more than one thing. Dr. Pasquale likes to say that he has taken the “P” out of POAG; grouping glaucoma by African-American race, estrogen deficiency, mitochondrial stress and endoplasmic reticulum stress, for example. “But I found that for any one patient, it’s hard to define those mechanisms, and patients probably have multiple mechanisms, so now I’m saying let’s just let the disease define itself,” he says. “Let the visual field be a read-out of the pattern of loss.” With NIH funding, he says that he and his colleagues are looking at visual defects and using artificial intelligence to turn them into a linear equation that quantifies the different regional patterns of loss. With 14 different patterns now identified, the next step is mapping these to the 120 genes they’ve identified as being associated with glaucoma. “Using two hospital-based repositories, we give everybody a genetic risk score,” Dr. Pasquale explains. The research also has an environmental component, which could further inform risk factors. By defining glaucoma in terms of disease process and establishing genetic links to visual defects like para-central loss, superior defects and inferior defects, Dr. Pasquale believes this establishes a more informed epidemiological framework. “We’re hopeful that we’ll be able to stratify more people, not by IOP, but by genetic risk score,” he says. The findings may do more than turn NTG on its head; they could reframe how POAG is classified, paving the way for targeted therapies. “The semantics issue is important,” says Dr. Pasquale. “We’re evolving from ‘low-tension’ to ‘normal-tension’ to everything else being POAG, so let’s take the ‘P’ out of it and figure it all out so we can have a precision-based medicine approach to the disease.” |
Another theory holds that NTG eyes may have a demographically different tolerance and/or genetic susceptibility to an IOP within the normal limit, according to Dr. Mohammadreza. “In addition, a large percentage of older NTG patients suffer from Alzheimer’s disease,” he adds.
The Alzheimer’s link has piqued physicians’ interest in the vasoprotective and neuroprotective treatment strategies, to which Dr. Netland referred earlier. “But, as clinicians, we have to go with what we know is effective, and IOP already does have this role,” says Dr. Mohammadreza.
Myopia has also been linked to NTG, but as they say, sometimes a cigar is just a cigar. Robert T. Chang, MD, assistant professor of ophthalmology at Stanford’s Byers Eye Institute, explains that, “myopia can be associated with elongation of the eye and resultant tilting and torsion of the optic nerve head, causing visual field defects that may resemble glaucomatous damage, but are simply the result of myopia, and not glaucoma,” as he noted in the May 2017 issue of Review.
The Hypertension Connection
Pinpointing associations with NTG may not change current treatment interventions; however, the hypertension/hypotension correlation to NTG is enough to compel some glaucoma specialists to take an active interest in a patient’s hypertensive medication regimen. “I always bring that topic up with my NTG patients, particularly those who are progressing despite a low IOP,” says Dr. Seibold. “If they seem to be at their target IOP, I’ll ask if they have systemic hypertension. Sometimes they’re overtreated to the point where their systolic blood pressure dips into the 90s. That can be detrimental, because now you’re lowering the ocular perfusion pressure,” he notes.
Just as the home use of tonometers may uncover off-hour IOP spikes, the use of Holter monitors has uncovered blood pressure readings a physician wouldn’t see in the office. “Holter monitoring can be a really nice thing because you get a 24-hour record of what’s happening with the patient’s cardiovascular parameters, and that can lead to tremendously helpful insights in some patients,” says Dr. Netland.
It may come down to a log of blood pressure measurements and a conversation with the patient’s primary care doctor, asking: “Can we [adjust blood pressure medications] to the benefit of higher ocular perfusion pressure?” Dr. Seibold suggests.
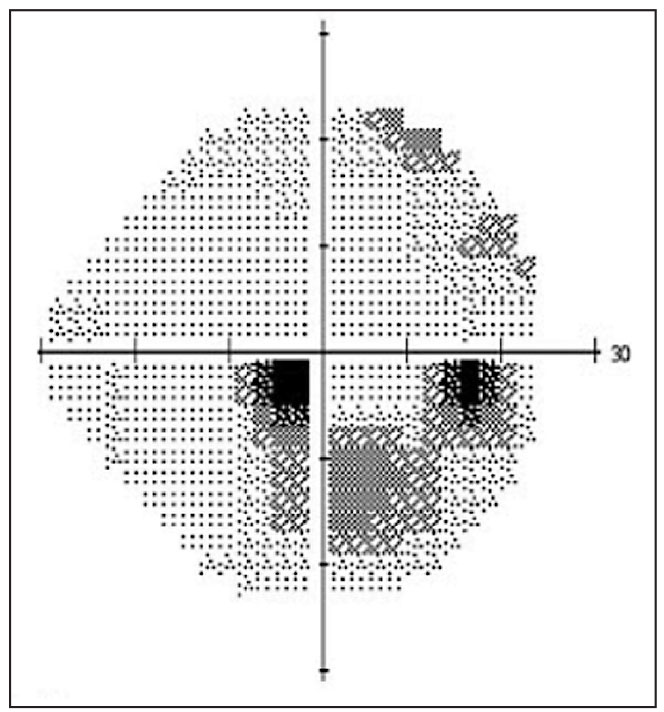 |
| Paracentral visual field loss in an eye with normal-tension glaucoma. |
Next Steps in NTG
“Overall, NTG has a fairly intractable clinical course,” says Dr. Netland. “But you can still reduce the rate of progression significantly, so that’s a very positive thing for patients. They’re not likely to go blind on their treatment, so there are many positives here.” That’s not to say that he doesn’t want to push the envelope for more effective treatments.
“Dropping the pressure by 30 percent doesn’t stop NTG,” Dr. Pasquale says of current interventions. “It still progresses. We need to explore alternative paradigms of treatment.” He says that the need for more aggressive treatments is evident for some patients, and he and his colleagues are working toward figuring out who those patients are. Today, he says, “We give a one-size-fits-all answer and say, ‘Most people with glaucoma, they’re going to do OK.’ But that’s disingenuous when we can say out of the other side of our mouth: ‘It’s the leading cause of blindness.’ Clearly not everybody is doing OK. We really need to be able to do a better job of forecasting so we can figure out who to be more aggressive with in using our current tools—until we get better tools.
Dr. Seibold is a consultant for New World Medical. Dr. Pasquale is a recipient of grant support from the National Eye Institute, and The Glaucoma Foundation Research to Prevent Blindness. He is a consultant for Twenty Twenty, Eyenovia and Skye Biosciences. Dr. Mohammadreza and Dr. Netland report no financial interests related to this article.
1. Mallick J, Devi L, Malik PK, et al. Update on normal tension glaucoma. J Ophthalmic Vis Res 2016;11:204-8.
2. Collaborative Normal-Tension Glaucoma Study Group: Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998;126:487-497.
3. Krupin T, Liebmann JM, Greenfield DS, et al. Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study [published correction appears in Am J Ophthalmol 2011 Jun;151:1108]. Am J Ophthalmol 2011;151:671-681.
4. Killer HE, Pircher A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye (Lond) 2018;32:924-930.



