It has been estimated that one in 150 people in the United States have severe visual impairment due to a retinal condition, ranging from extremely rare “orphan” hereditary retinal degenerative diseases to diabetic retinopathy. Individuals diagnosed with retinitis pigmentosa or Leber’s congential amaurosis have a poor visual prognosis and potential treatments are limited. For decades scientists and ophthalmologists have sought ways to stop the progression of these diseases though, until recently, without success. In the past five years, gene therapy has emerged in ophthalmology as a new approach in the treatment of many retinal disorders that are considered incurable.
A Setback, Then Progress
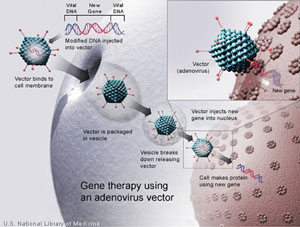
In the 1990s, gene therapy was a promising, novel approach to treat human disease by infecting a patient’s cells, typically with a viral vector containing a gene that encodes a therapeutic protein (See Figure 1). However, after the death in 1999 of a patient treated for ornithine transcarbamylase deficiency and continued issues with immunogenicity, carcinogenicity, vector manufacturing and small patient populations, many physicians and scientists lost hope for gene therapy.1
Learning from these initial setbacks, investigators narrowed their targets to rare monogenic disorders and focused on conditions localized to defined anatomic areas, such as the eye and the brain. Most recently, intravenous delivery of an adenovirus-associated virus (AAV) vector expressing human factor IX has resulted in factor IX transgene expression at sufficient levels to improve the bleeding phenotype in six individuals affected with hemophilia B; the treatment also was safe.2 This work has helped to reignite the promise of gene therapy for systemic human disease.
|
The eye, in particular, is an attractive target for gene therapy for several reasons. The eye is one of the few immunologically privileged sites in the body, so the gene vectors used are unlikely to cause a systemic immune response. Given the defined volume of the eye, small amounts of viral vectors may be all that are necessary to achieve therapeutic effects—likely to be a positive for reducing the risk of toxicity and increasing the likelihood of being able to manufacture quantities of vector sufficient to treat the retina. The eye also allows for localized treatment without intravenous delivery, thus decreasing the chance of systemic absorption and toxicity. Finally, the effects of localized ocular treatments can be easily observed and monitored for efficacy and safety, something that cannot be readily done with systemic conditions. With these advantages in using the eye as a target for gene therapy, and the continued understanding of gene mutations and their role in retinal diseases, investigators are actively determining the potential for gene therapy in different conditions (See Table 1).
Historically, gene therapy has been considered a treatment modality used for monogenetic heredodegenerative diseases. For instance, investigators are using gene therapy to target mutations in the RHO gene to halt the progression of retinitis pigmentosa and the MY07A gene in Usher’s syndrome.3,4 Animal models suggest that gene therapy can be used to treat diseases such as achromatopsia, Stargardt’s disease and X-linked retinoschisis.5,6 There may even be a role for gene therapy in multifactorial degenerative diseases of the retina, like diabetic retinopathy and age-related macular degeneration.7-9
To date, 12 early, Phase I clinical trials using gene therapy have been initiated for various retinal diseases such as exudative AMD, choroideremia, autosomal recessive RP and Stargardt’s disease (See Table 1).6 Leber’s congenital amaurosis (RPE65 mutation) is currently in advanced development with patients being evaluated in a Phase III clinical trial.10 All but one of these clinical studies uses an adeno-associated viral vector. Adeno-associated viruses are non-pathogenic, small viruses with one single strand of DNA from the parvovirus family. AAV vectors have a low risk of insertional mutagenesis, especially for the post-mitotic tissue such as the retina. These viruses have been shown to insert genetic material into specific sites with a high rate of reproducibility, and human studies have found AAV to persist in humans for more than three and a half years.11 AAV-vector-mediated gene delivery has been safely used in more than 20 clinical trials for diseases such as Parkinson’s disease, cystic fibrosis and hemophilia.2,12,13
| ||||||||||||||||||||||||||||||||||||||||
Recently, there have been tremendous advances in our understanding of the etiology of AMD in an effort to target treatment to the underlying cause of the disease. Many investigators postulated that the disease is a result of accumulative damage from inflammation, toxic by-products and oxidative damage that lead to destruction of the choroid, RPE and retina. Investigators have been evaluating a variety of ways to treat AMD, ranging from neuroprotection to preserve photoreceptors and the RPE to suppression of the inflammatory cycle via complement inhibitors, glucocorticoids and immunosuppressive agents.18-22
Enter Biologics
One major breakthrough occurred in 2006, when ranibizumab (Genentech), a monoclonal antibody fragment that binds and inhibits vascular endothelial growth factor A, was approved by the FDA for the treatment of exudative AMD.23 Currently, the only way to successfully treat neovascular AMD requires frequent injections of a biopharmaceutical, such as ranibiz-umab, pegaptanib (Genentech) or aflibercept (Regeneron), that require patients to undergo the risk of multiple invasive procedures and the significant economic burden relating to the need for frequent treatments. Since the approval of ranibizumab, scientists have been working on prolonging the effects of biologics for treating retinal conditions. Local gene transfer offers the possibility of targeted, sustained delivery of biologics to the retina.
One of the first clinical trials investigating gene therapy for the treatment of exudative AMD was done by Peter Campochiaro, MD, and associates. They treated 28 patients with advanced exudative AMD with a single intravitreal injection of an adenoviral vector expressing human pigment epithelium-derived factor (AdPEDF.11). They observed antiangiogenic activity resulting in the inhibition of lesion growth. In 25 percent of the patients in the study there was mild, transient intraocular inflammation, thought to be secondary to the adenovirus vector. In the high-dose group of patients, there was no further growth of the neovascular complex after treatment, with patients being followed for a year.24 This was suggestive that antiangiogenic activity could be observed for several months after gene transfer.
Coupling the sustained local effect of proteins expressed by gene therapy with the known effects of anti-VEGF agents, Genzyme developed a new AAV gene therapy that expresses a modified soluble Flt1 receptor or VEGR receptor 1 (VEGFR-1). The idea is that expression of the receptor will limit the proangiogenic activities of VEGF after a single intravitreal injection for a sustained period of time. Pre-clinical animal models suggest that the gene products of AAV2-sFLT01 bind with high affinity to VEGF and inhibit its neovascular activity with expression lasting for at least 18 months. AAV2-sFLT01 is currently involved in a Phase I safety clinical trial in advanced exudative AMD.25,26 AAV2-sFLT01 is administered via an intravitreal injection.
Numerous published studies suggest that genetic polymorphisms in the complement cascade are associated with an increased risk of developing AMD. Variants in complement factors H (CFH), 3 (C3), B (CFB) and 2 (C2) have been shown to be a driving force in the inflammatory component of AMD in various populations worldwide.27-32 These genetic association studies provide the foundation for the new hypothesis on the pathogenesis of AMD. Polymorphisms in a complement regulator or activator are thought to result in overactivation of the complement cascade, leading to either: sublytic levels of membrane attack complex (MAC) on cells, promoting the release of VEGF and mitogenesis, resulting in exudative AMD; or lytic levels of MAC deposited on cells, initiating cell lysis, resulting in geographic atrophy. Elevated levels of MAC have been found in choroidal blood vessels and RPE/Bruch’s membrane in patients with AMD.33
One example, in preclinical study, of an approach for modifying the complement system is being done at Hemera Bioscience. HMR59 is an AAV2 vector that expresses soluble CD59. CD59 is a naturally occurring protein anchored to plasma membranes that protects cells from complement damage by blocking the formation of membrane attack complex. The soluble form of CD59, sCD59, is not membrane-bound, allowing it to freely circulate and block MAC formation. Pre-clinical testing in the laser choroidal neovascularization mouse model shows that prior intravitreal injection of HMR59 inhibits the formation of CNV by 56 percent.34-36
In summary, ocular gene therapy appears to be a promising new approach to the treatment of retinal diseases. There are currently more than 30 pre-clinical studies or clinical trials investigating the potential benefits of gene therapy for retinal conditions. Gene therapy appears to be well-tolerated and effective in treating retinal disorders as evidenced by early clinical trials. The localized nature of these conditions to the eye, the small amounts of viral vector that appear to be all that are necessary to show treatment effects, and the immune privilege of the eye may prove advantageous relative to treating systemic conditions with gene therapy. Finally, sustained delivery of biologics may be attainable with a single treatment, something the entire retinal community will embrace, given the chronic nature of these targeted retinal diseases. REVIEW
The authors are at the New England Eye Center, Tufts University School of Medicine. Dr. Bryant is a vitreoretinal fellow; Dr. Duker is a professor and chairman of the center; and Dr. Reichel is a professor and director of the Vitreoretinal Service. Drs. Reichel and Duker own equity in Hemera Biosciences.
1. Mullard A. Gene therapies advance towards finish line. Nature Reviews 2011;10:719-720.
2. Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-Associated Virus Vector-Mediated Gene Transfer in Hemophilia B. N Engl J Med 2011;Dec 22;365(25):2357-65.
3. Mao H, Gibbs D, Lillo C, et al. AAV delivery of wild-type rhodopsin preserves retinal function in a mouse model of autosomal dominant retinitis pigmentosa. Hum Gene Ther 2011; 22(5):567-75.
4. Hashimoto T, Gibb D, Lillo C, et al. Lentiviral gene replacement therapy of retinas in a mouse model for Usher syndrome type 1B. Gene Ther 2007;14(7):584-94.
5. Carvalho LS, Xu J, Pearson RA, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet 2011;20(16):3161-75.
6. Liu M, Tuo J, Chan C,. Gene therapy for ocular disease. Br J Ophthalmol 2011;95(5):604-12.
7. Prentice H, Biswal M, Dorey C, et al. Hypoxia-regulated retinal glial cell-specific promoter for potential gene therapy in disease. Invest Ophthalmol Vis Sci 2011;52(12):8562-70.
8. Stieger K, Cronin T, Bennett J, et al. Adeno-associated virus mediated gene therapy for retinal degenerative diseases. Methods Mol Biol 2011;807:179-218.
9. Campochiaro P. Gene transfer for neovascular age-related macular degeneration. Hum Gene Ther 2011;22(5):523-9.
10. Simonelli F, Maguire A, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 2010;18(3):643-50.
11. Roy K, Stein L, Kaushal S. Ocular gene therapy: An evaluation of recombinant adeno-associated virus-mediated gene therapy interventions for the treatment of ocular disease. Hum Gene Ther 2010;21(8):915-27.
12. Rodnitzky R. Upcoming treatments in Parkinson’s disease, including gene therapy. Parkinsonism Relat Disord 2012;18 Suppl 1:S37-40.
13. Mueller C, Flotte T. Gene Therapy for cystic fibrosis. Clin Rev Allergy Immunol 2008;35(3):164-78.
14. Cai X, Conley S, Naash, M. RPE65: Role in the visual cycle, human retinal disease, and gene therapy. Ophthalmic Genet 2009;30(2):57-62.
15. Bainbridge JW, Smith AJ, Barker SS, Robbie S, et al. Effect of gene therapy on visual function in Leber’s congenital. N Engl J Med 2008;358(21), 2231–2239.
16. Maguire A, Simonelli F, Pierce E, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240-8.
17. Hauswirth W, Aleman T, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther 2008;19(10):979-90
18. Thorton J, Edwards R, Mitchell P, et al. Smoking and age related macular degeneration: A review of association. Eye 2005;19:935-944.
19. Seddon J, Cote J, Rosner B. Progression of age-related macular degeneration: Association with dietary fat, transunsaturated fats, nuts, and fish intake. Arch Ophthalmol 2003;121:1728-37.
20. Yehoshua Z, Rosenfeld PJ, Albini TA. Current clinical trials in dry AMD and the definition of appropriate clinical outcomes measures. Semin Ophthalmol 2011 May;26(3):167-80.
21. Ding X, Patel M, Chan C. Molecular pathology of age-related macular degeneration. Prog Retin Eye Res 2009; 28:1-18
22. Dunaief J, Dentchev T, Ying G, et al. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol 2002;120:1435-1442.
23. Rosenfeld P, et al for the MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-1431.
24. Campochiaro PA, Nguyen QD, Shah SM, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: Results of a phase I clinical trial. Hum Gene Ther 2006;17(2):167-76.
25. Lukason M, DuFresne E, Rubin H, et al. Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther 2011;19(2):260-5.
26. Maclachlan T, Lukason M, Collins M, et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther 2011;19(2):326-34.
27. Postel EA, Agarwal A, Caldwell J, et al. Complement factor H increases risk for atrophic age-related macular degeneration. Ophthalmology 2006;112:1504-1507.
28. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308(5720):385-389.
29. Edwards AO, Ritter R, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science 2005;308(5720):362-364.
30. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. PNAS 2005; 02(20):7227-7232.
31. Maller JB, Fagerness J, Reynolds RC, et al. Variation in complement factor 3 is associated with risk of age related macular degeneration. Nature Genetics 2007;39(10):1200-1201.
32. Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age related macular degeneration. Nature Genetics 2006;38(4):458-462.
33. Mullins RF, Dewald AD, Streb LM, et al. Elevated membrane attack complex in human choroid with high risk component factor H genotypes. Experimental Eye Research 2011;93:565-567.
34. Bora N, Kaliappan S, Jha P. et al. CD59, a complement regulatory protein, controls choroidal neovascularization in a mouse model of wet-type age-related macular degeneration. J Immunol 2007;178(3):1783-90.
35. Ramo K, Cashman S, Kumar-Singh R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest Ophthalmol Vis Sci 2008;49(9):4126-36.
36. Cashman S, Ramo K, Kumar-Singh R. A non membrane-targeted human soluble CD59 attenuates choroidal neovascularization in a model of age related macular degeneration. PLoS One 2011 Apr 28;6(4):e19078.