For a long time, laser was the first-line option for treating patients
with diabetic macular edema and, though it was effective, it could be
destructive, as well. Currently, retinal experts say you should
probably add anti-vascular endothelial growth factor drugs to your
arsenal when dealing with DME—but you should also keep your laser handy
for certain situations. Here, ophthalmologists who deal with DME every
day explain how the two treatment modalities fit together.
What the Studies Say
In terms of agents that are currently available, physicians began to add anti-VEGF drugs such as ranibizumab (Lucentis) and bevacizumab (Avastin) to their treatment regimen after seeing the results of several key randomized studies.
In a study from the RESTORE study group in Sydney, Australia, researchers compared ranibizumab alone, as well as ranibizumab combined with laser, to the previous gold standard, laser monotherapy.1 In RESTORE, 345 patients with visual impairment due to DME were randomized to one of the three treatments. Ranibizumab alone (plus sham laser) was given for three months, then on a p.r.n. basis afterward. Laser was given at baseline and then as needed.
Ranibizumab alone, as well as ranibizumab combined with laser, were superior to laser alone in improving the mean change in best-corrected acuity letter score from the baseline visit to months one through 12 (+6.1 and +5.9 letters vs. +0.8 letters for laser alone; p<0.0001). At one year, 22. 6 percent of ranibizumab patients had a best-corrected letter score of at least 15, and 53 percent had a best-corrected letter score level greater than 73 (20/40 Snellen equivalent); for the ranibizumab plus laser group, the proportion was 22.9 percent and 44. 9 percent, respectively. However, for laser alone, only 8.2 percent of the patients had a score of at least 15 letters and 23.6 percent had a letter score level greater than 73.
 In RESTORE, researchers reported that the mean central retinal thickness
was significantly reduced from baseline with ranibizumab and
ranibizumab plus laser. Patients received a mean of seven ranibizumab/sham injections over 12 months. An increase in intraocular pressure
occurred in one patient each in the ranibizumab arms. Ranibizumab
monotherapy or ranibizumab combined with laser wasn’t associated with an
increased risk of cardiovascular or cerebrovascular events.
In RESTORE, researchers reported that the mean central retinal thickness
was significantly reduced from baseline with ranibizumab and
ranibizumab plus laser. Patients received a mean of seven ranibizumab/sham injections over 12 months. An increase in intraocular pressure
occurred in one patient each in the ranibizumab arms. Ranibizumab
monotherapy or ranibizumab combined with laser wasn’t associated with an
increased risk of cardiovascular or cerebrovascular events.
Similarly, the BOLT study from the United Kingdom found bevacizumab to be effective against DME when compared to laser alone, with patients in the drug group having a 5.1 times greater chance than those in the laser group of gaining 10 or more ETDRS letters over 12 months.2 Also, a recent randomized two-year, Phase II/ III trial of pegaptanib sodium for the treatment of DME found the drug to be effective when compared to sham injection and subsequent (after 18 weeks) laser.3
The READ-2 study from the Wilmer Eye Institute and the READ-2 Study Group found that, though intravitreal injections of ranibizumab were a great addition to a physician’s DME treatment regimen, the clinician could combine them with focal or grid laser and decrease the number of injections while keeping the good results.4 In READ-2, 126 patients were randomized 1:1:1 to receive 0.5-mg ranibizumab initially at months one, three and five (group 1); focal or grid laser at baseline and at month three if needed (group 2); or a combination of ranibizumab and focal or grid laser at baseline and month three (group 3). After six months, most patients in all of the groups were treated solely with ranibizumab because they met the retreatment criteria.
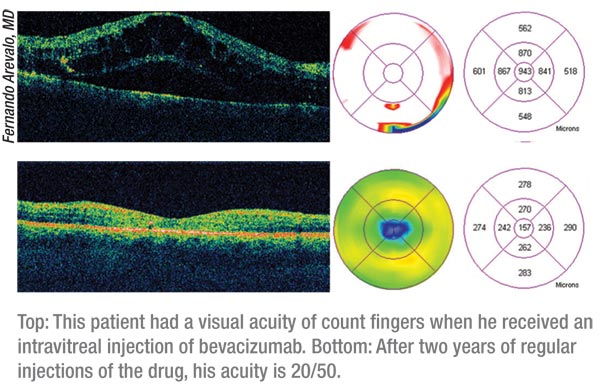 |
At six months, the mean improvement in best-corrected vision in group 1
was 7.4 letters (n=33); in group 2 it was 0.5 letters (n=34); and in
group 3 (n=34) it was 3.8 letters. At two years, the mean improvement
was 7.7 (group 1), 5.1 (group 2) and 6.8 letters (group 3). In groups
1, 2 and 3, the percentage of patients who gained three lines or more of
vision was 21, 0, and 6 percent at six months, vs. 24, 18 and 26
percent at two years. At two years, 45 percent of group 1, 44 percent
of group 2 and 35 percent of group 3 had 20/40 or better Snellen
equivalent best-corrected vision. Mean foveal thickness at two years
was 340 ìm (group 1), 286 ìm (group 2), and 258 ìm (group 3). The
percentage of patients with a center subfield thickness of 250 ìm or
less was 36 percent (group 1), 47 percent (group 2) and 68 percent
(group 3). The researchers point out that combining the drug with laser
reduced both the amount of residual edema and the mean number of
injections the patients had to endure: a mean of 5.3 injections for
Group 1, 4.4 for Group 2, and just 2.9 for Group 3 after 18 months.
New Practice Patterns
New Practice Patterns
The results of the research studies as well as ophthalmologists’ clinical impressions have shaped current practice patterns. Some incorporate laser into most of their treatments, while others use mostly anti-VEGF agents and add laser only in certain cases.
“We’re just starting to understand what it means to treat diabetic macular edema,” says Phoenix, Ariz., ophthalmologist Pravin Dugel.“We’re on the cusp of understanding which combination of treatments is proper for which stage of the disease. It’s not a one-size-fits-all type of thing because DME is progressive.It’s a long-term, chronic disease, and I know that laser will still have a role, as will steroids and anti-VEGF agents.”
Diana Do, MD, of the Wilmer Eye Institute, says the advent of anti-VEGF agents has changed her approach to DME. “My first-line treatment for center-involved DME is intravitreal agents,” she says. “I’ve decreased my use of laser because multiple clinical trials have shown that intravitreal anti-VEGF agents result in superior visual acuity outcomes compared to focal and grid laser. However, for patients who have non-center-involved macular edema, I still feel that laser is an option.”
Dr. Dugel says the multi-stage nature of DME lends itself to multiple
treatment approaches. “In the beginning, DME starts out in a focal
manner,” he says. “At the outset, a leak occurs in a specific area that
may cause the edema we see and make the vision worse with time. In
that early stage, if we can see an area of leakage and it’s away from
the center of vision, it makes sense to use laser photocoagulation. With
laser, you might be able effect a long-term treatment, and, if you can
use the laser without damaging central vision, you’ve avoided having the
patient depend on a particular drug for an extended period.
Unfortunately, we often see patients later than this. And, as the
disease progresses, it comes close to the center of vision, and you
don’t want to destroy the center with laser. At that point, there may
be some areas where you can do laser while also administering
intravitreal anti-VEGF injections to control the other areas. And, if
it’s very late-stage, we might use several types of agents and possibly
laser on certain areas.” For his treatment protocol, he says he prefers
to approximate the approach known as “Protocol-I” from the Diabetic
Retinopathy Clinical Research Network’s study of DME.5 “I administer
intravitreal injections on a monthly basis until I reach some stability
in the disease,” he says. “I then re-inject if there’s worsening of
vision or macular thickening.”
A somewhat controversial treatment is the use of sub-threshold micropulse laser for DME. Some surgeons feel they need to see more data on it before they put it to use, while others think it’s a beneficial addition to a treatment plan. Edoardo Midena, MD, chairman of the ophthalmology department of the University of Padova in Italy, has studied the effects of subthreshold laser photocoagulation in his practice and thinks it has potential.
“The concept of sub-threshold diode laser—and possibly sub-threshold yellow laser in the future—is to be able to treat the retina and obtain a reduction in the appearance of DME without inducing side effects in the neural part of the retina,” he says. “This means there would be no changes in retinal sensitivity like those that occur with standard laser photocoagulation and manifest themselves as scotomas in the visual field.
“Sub-threshold means that, when you perform the laser treatment, you don’t see any laser spots on biomicroscopy or, in our experience, on fluorescein angiography, but you still get a reduction in the appearance of DME,” Dr. Midena adds. In a prospective, randomized study at his practice, Dr. Midena performed sub-threshold micropulse laser on 32 DME eyes using an Iridex diode laser as well as standard laser treatment (modified ETDRS approach) with a green laser. The sub-threshold treatment’s laser settings are a spot size of 125-ìm diameter, 750 mW power and 200 msec duration.
At one year, both groups maintained their visual acuity and had the same level of reduction in retinal thickness. Dr. Midena says the difference came in terms of retinal sensitivity: In the sub-threshold group, there was no change, or an improvement in sensitivity on microperimetry compared to the standard group, in which there was a decrease.
In the real world of clinical practice, Dr. Midena says his approach is a mix of modalities. “Micropulse isn’t a treatment for all diabetics, because massive macular edema can’t be treated with just one therapeutic system,” he says. “When the retina is very thick, as in more than 400 ìm, it’s better to inject a substance that can decrease it, such as anti-VEGF agents or steroids, for a very fast reduction in thickness. The most important problem with these drugs, however, is that you have to continuously inject them. So, for DME we’d consider an initial injection followed by maintenance with sub-threshold micropulse laser about 15 days after the injection.”
Dr. Do, however, thinks more investigation is necessary into sub-threshold laser. “Several physicians are investigating it,” she says. “But at this time I think an anti-VEGF agent is more effective than a laser.”
Future Therapies
A somewhat controversial treatment is the use of sub-threshold micropulse laser for DME. Some surgeons feel they need to see more data on it before they put it to use, while others think it’s a beneficial addition to a treatment plan. Edoardo Midena, MD, chairman of the ophthalmology department of the University of Padova in Italy, has studied the effects of subthreshold laser photocoagulation in his practice and thinks it has potential.
“The concept of sub-threshold diode laser—and possibly sub-threshold yellow laser in the future—is to be able to treat the retina and obtain a reduction in the appearance of DME without inducing side effects in the neural part of the retina,” he says. “This means there would be no changes in retinal sensitivity like those that occur with standard laser photocoagulation and manifest themselves as scotomas in the visual field.
“Sub-threshold means that, when you perform the laser treatment, you don’t see any laser spots on biomicroscopy or, in our experience, on fluorescein angiography, but you still get a reduction in the appearance of DME,” Dr. Midena adds. In a prospective, randomized study at his practice, Dr. Midena performed sub-threshold micropulse laser on 32 DME eyes using an Iridex diode laser as well as standard laser treatment (modified ETDRS approach) with a green laser. The sub-threshold treatment’s laser settings are a spot size of 125-ìm diameter, 750 mW power and 200 msec duration.
At one year, both groups maintained their visual acuity and had the same level of reduction in retinal thickness. Dr. Midena says the difference came in terms of retinal sensitivity: In the sub-threshold group, there was no change, or an improvement in sensitivity on microperimetry compared to the standard group, in which there was a decrease.
In the real world of clinical practice, Dr. Midena says his approach is a mix of modalities. “Micropulse isn’t a treatment for all diabetics, because massive macular edema can’t be treated with just one therapeutic system,” he says. “When the retina is very thick, as in more than 400 ìm, it’s better to inject a substance that can decrease it, such as anti-VEGF agents or steroids, for a very fast reduction in thickness. The most important problem with these drugs, however, is that you have to continuously inject them. So, for DME we’d consider an initial injection followed by maintenance with sub-threshold micropulse laser about 15 days after the injection.”
Dr. Do, however, thinks more investigation is necessary into sub-threshold laser. “Several physicians are investigating it,” she says. “But at this time I think an anti-VEGF agent is more effective than a laser.”
Future Therapies
There are therapies not yet approved in the United States that may be effective when added to the current options, say surgeons.
 |
However, the drawbacks to steroids remain the induction of cataract and increase in IOP. In FAME, 80 percent of phakic patients in the 0.2-ìg group needed cataract surgery vs. 27.3 percent of controls. In terms of IOP, 18.4 percent of the study group had an IOP greater than 30 mmHg at some point in the study vs. 4.3 percent of controls, and 4.8 percent of the implant group needed glaucoma surgery compared to just 0.5 percent of control patients.
Tom Ciulla, MD, of Indianapolis, has taken part in the study of Iluvien, and says he thinks it might have a niche for certain DME patients. “It appears to be very efficacious,” he says. “I think sustained-release steroids will be beneficial because they allow for a single therapy as opposed to multiple repeated intravitreal injections. However, you might not want to use it as a first-line treatment in someone with mild edema. But if the patient has severe edema and has lost a lot of vision, I think the benefits will outweigh the steroid risks.” Dr. Do says a sustained-release steroid might be useful in pseudophakic patients or those with glaucoma who have already had a tube shunt or trabeculectomy procedure, which might mitigate the steroid risks.
Also in the trial pipeline is the Ozurdex implant (Allergan), which releases dexamethasone over time. “It’s currently in trials, and should work for DME as well,” says Dr. Ciulla. “It has a shorter duration of action than the fluocinolone implant, which could be a positive and negative, because if someone has a pressure spike with a shorter-acting sustained-release device, sometimes you can just wait it out with eye drops. On the other hand, it would require more injections compared to a longer-acting implant.”
• VEGF Trap-Eye (Regeneron). VEGF Trap-Eye is an anti-VEGF compound designed to be delivered intravitreally, and is being studied vs. macular laser in DME in the DaVinci study. In the study, researchers say the drug produced significant gains in best-corrected acuity at 24 weeks and sustained or improved on them throughout a year, including in one group that received treatment every other month. They say the drug is well tolerated, with the most frequent ocular adverse events being conjunctival hemorrhage, eye pain, ocular hyperemia and increased IOP. (Tolentino M, et al. IOVS 2011;52:ARVO E-Abstract 6646)
Though anti-VEGF agents are effective for macular edema, Dr. Dugel doesn’t think frequent injections are the ultimate answer. “The vast majority of DME patients need multiple injections over time,” he muses. “Is this sustainable? In my opinion, it’s not. I think a sustained-release drug, used in combination with our current therapies, will ultimately help make this treatment sustainable.”
1. Mitchell P, Bandello F, Schmidt-Erfurth U. The RESTORE study:Ranibizumab monotherapy or combined with laser versus laser monotherapy for DME. Ophthalmology 2011;118:4:615-25.
2. Michaelides M, Kaines A, Hamilton RD. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology 2010;117:6:1078.
3. Sultan MB, Zhou D, Loftus J, et al. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011;118:6:1107-18.
4. Nguyen QD, Shah SM, Khwaja AA. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117:11:2146-51.
5. Elman MJ, Aiello LP, Beck RW, et al. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular Edema. Ophthalmology 2010;117:6:1064-1077.



