FUNDUS AUTOFLUORESCENCE (FAF) IMAGING, A FAST AND non-invasive technique developed over the last decade, utilizes fluorescent properties of lipofuscin to study the health and viability of the retinal pigment epithelium/photoreceptor complex.
Lipofuscin (LF) is a mixture of autofluorescent pigments that accumulate in post-mitotic cells throughout life. In the RPE, LF granules accumulate in the lysosomal compartment mainly as a by-product of constant phagocytosis and incomplete digestion of discs and their retinoid content shed from PR outer segments.1,2,3 Formation of these fluorophores is dependent upon a normal visual cycle4 and LF is therefore an indirect marker of metabolic activity between PR OS turn over and RPE phagocytosis ability. Abnormal accumulation of LF is a common finding in inherited retinal disease and age-related macular degeneration. It is still a matter of debate, however, whether or not LF has role in the pathogenesis of disease. The distribution and intensity of FAF may provide insight into the sequence of events that leads to retinal damage and may help elucidate pathophysiological mechanisms.
Until recently, only histological studies were available to document LF content in the RPE. Major limitations included the paucity of samples, usually obtained at late stages in case of retinal diseases and often confounded by fixation artifact, the absence of direct correlation with fundus changes, and the lack of sequential examination. Different techniques have become available in the past decade to record FAF in vivo however.5,6,7 The investigators who developed the first non-invasive system using a fundus spectrophotometer5 showed that in vivo when eyes are excited with a short wavelength light, FAF is derived mainly from RPE LF granules with a broad emission spectrum from 500 to 750 nm and a peak at about 630 nm.6 Confocal Scanning Laser Ophthalmoscope (cSLO) allows the spatial distribution of FAF over large retinal areas to be documented, such that correlations may be made between FAF and pathologic features observed on fundus examination.7,8,9 The use of a confocal system and a monochromatic light allows high spatial resolution imaging of a sharp image in a selected plane and avoids lens fluorescence. To study FAF, cSLO usually utilizes an argon blue laser of 488 nm, also used for fluorescein angiography, and a barrier filter with a short wavelength cut off at 495 nm to separate the excitation from the emitted fluorescent light. Better pictures are obtained after dilation of the pupil. A focus device allows ametropic corrections up to ±12 D. To amplify the autofluorescence signal, single images are usually averaged after eye movement correction. This may be difficult in cases of nystagmus or poor fixation.
It is difficult to quantify autofluorescence intensity accurately using current cSLO technology, and any attempt at accurate inter-individual comparisons of intensity must allow for variations in lens absorption, pupil dilation and variation of the incident laser light intensity. Some authors have favored topographical variations, while others have developed grey scale measurement to compare FAF changes between subjects, with10 or without11 calibration.
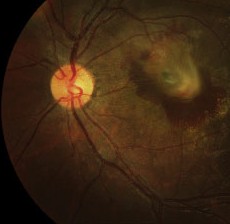
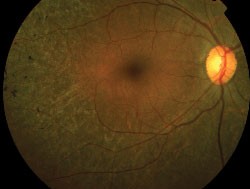
The normal FAF pattern is consistent with histological data of LF distribution.7,12-14 It is higher in the parafoveal area and decreases toward the periphery. This parallels rod photoreceptor density and may reflect metabolic load of the RPE cells. It dips in the fovea due partially to absorption of the short wavelength light by melanin and luteal pigment and partly due to decreased LF content in that area.14 There is reduced autofluorescence at the optic disc due to the absence of RPE, and at blood vessels due to the masking of the RPE by the overlying blood column.
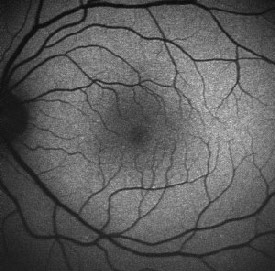 |
| Normal fundus autofluorescence. Note the relative lack of autofluorescence in and around the fovea, and the complete absence of auto-fluorescence at the optic disk (where there is no RPE) and corresponding to the the large and moderate-sized retinal vessels (where the signal is obscured by the blood column). |
The following discussion briefly outlines the major diagnostic and prognostic applications of FAF in retinal disease.
FAF Imaging in Retinal Disease
• Age-related macular degeneration. Several reports in the literature document FAF changes in AMD in relation to retinal function and prognosis.
Drusen in AMD are usually associated with normal FAF when analyzed with cSLO,9,15,16 whereas in juvenile drusen such as in familial-dominant drusen15 or PXE,17 for example, this is not the case. However, large soft foveal drusen may represent a separate entity associated with increased FAF.16,18
Areas of hyperpigmentation on fundus examination of AMD are associated with focal increase of FAF, which might indicate increased metabolic activity of the PR/RPE complex.19 The same findings are observed with RPE detachments.8
Irregular FAF is usually present over well-established choroidal neovascular complexes and disciform scars and its analysis is important to assess PR/RPE integrity. However, AF may be intact for up to two years after the initiation of neovascularization. High FAF can extend beyond the edge of the lesion.8 Phagocytosis of debris derived from exudation may contribute to the accumulation of intracellular material.8
|
|
|
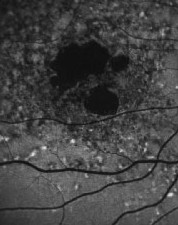
| Left eye of a 70-year-old patient with choroidal neovascular membrane. Note the decreased FAF associated with the disciform scar formation and hemorrhage and the heterogeneously increased FAF surrounding the lesion. |
|
|
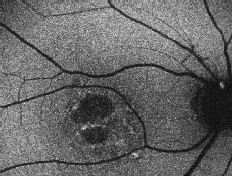 |
| Right eye of a 78-year-old patient with age-related macular degeneration including drusen, geographic atrophy and pigmentary changes. Note the sharp demarcation of the area of atrophy and the increased FAF at the edge of the lesion. |
Further longitudinal and in vitro studies are needed to solve these different issues. However, mapping of FAF variations in AMD appears to be of prognostic value and would serve to identify patients at risk for occurrence or enlargement of absolute scotomata and, thus, severe visual loss.
Inherited Retinal Dystrophies
FAF imaging is a valuable additional tool in phenotypic/ genotypic characterization and may identify patients who would benefit from PR rescue.
• Retinitis pigmentosa. Loss of peripheral FAF corresponds with the distribution of PR cell loss in RP.9 Adjacent areas of surviving retina can show increased FAF in absence of ophthalmic abnormalities. Macular edema, present in some RP cases, manifests initially as increased FAF. Chronic macular edema, in contrast, may result in a loss of PR and decrease in FAF.9
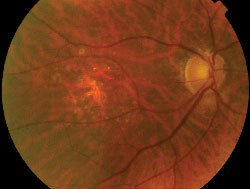
An abnormal parafoveal ring of increased FAF is present in some patients with RP and normal visual acuity.7,10,24 The diameter of this high-density ring is correlated with the Pattern Electroretinogram (PERG) P50 component, which indicates macular cone system function.10 Psychophysical studies, using fine matrix mapping, have shown that this ring demarcates different regions of sensitivity loss: photopic sensitivity is preserved inside the high-density ring with a gradient of photopic sensitivity loss over the region of increased FAF with severe loss outside the ring. Scotopic sensitivity losses are more severe and encroached within the ring.24 These observations suggest that mild rod macular dysfunction precedes significant LF accumulation and increased FAF.10 The significance of a similar ring in patients with impaired visual acuity is not known. It may be anticipated that the ring will constrict as the disease and macular involvement progress, but longitudinal studies are needed to investigate this proposal.
|
|
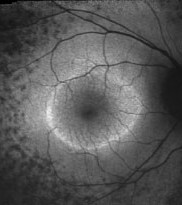 |
| Right eye of a 42-year-old patient with retinitis pigmentosa and 20/20 visual acuity. Note the parafoveal ring of increased FAF and decreased FAF outside the vascular arcades. |
Absent or very low FAF has been described early in the course of the disease of patients with RPE65 mutations26 and in the Rpe65 knock-out mice4 without photoreceptor cell death on OCT.26 This is a unique feature in retinal diseases, RPE65 mutations leading to a disruption of the visual cycle with absence of both all-trans retinal and 11-cis retinal and thus absence of LF formation. Similarly, a lack of FAF may occur in patients with mutations in other genes that disrupt the visual cycle, such as LRAT, but this remains to be demonstrated.26 This may direct genotypic analysis. In this subgroup of LCA, the use of ultra-high resolution OCT gives better information on PR integrity for future gene therapy approaches. FAF imaging would, however, be an easy way to monitor gene therapy efficacy, as restoration of gene function would lead to newly formed LF and potential FAF detection.
Other Retinal Dystrophies
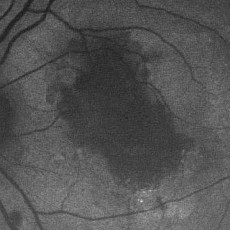
Distinctive FAF features in several other retinal dystrophies have been identified and are a useful and sensitive diagnostic tool, which may negate the need for fluorescein angiography. Macular dystrophies such as Stargardt/ fundus flavimaculatus, Best vitelliform, adult vitelliform dystrophy or maternally inherited diabetic disease and deafness are associated with focal increased FAF, which can be detected early in the course of the disease when fundus changes are subtle.9,11,15,27-30 Focal increases tend to increase with time and eventually give way to focally decreased FAF or PR/RPE atrophy. Stargardt disease has a variable phenotype, with cases limited to a well-demarcated decreased foveal FAF corresponding to central atrophy and few parafoveal hyperfluorescent flecks, or a more characteristic pattern with peripapillary sparing, decreased foveal FAF due to central atrophy, and hyperfluorescent flecks associated with areas of focal decreased FAF.31 The presence of a dark choroid can not be explained on the basis of increased FAF background and might be associated with accumulation of different fluorophores.27,28
|
|
|
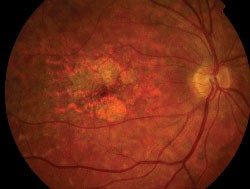
| Right eye of a 31-year-old patient with Stargardt/fundus flavimaculatus. Note the sharply demarcated central area of atrophy, the irregular hyper/hypo autofluorescence in the posterior pole and the sparing of the peripapillary area. |
Acquired Diseases
FAF imaging can be a useful tool in the detection of acquired retinal diseases, an aid to correlate fundus changes with physiopathology and help in therapeutic decisions, although few studies have been published and the literature is not comprehensive. Focal increased FAF is usually associated with an active process, as in central serous chorioretinopathy, at the site of leakage and in the area of retinal detachment32 or in idiopathic acute posterior multifocal placoid pigment epitheliopathy in the acute phase.33 Chronic lesions will usually result in decreased FAF due to secondary RPE/PR atrophy.
FAF imaging is a fast and non-invasive technique, which gives useful insight into PR/RPE metabolism. Cross-sectional and longitudinal studies are still needed to evaluate the prognostic value of specific changes and to obtain better phenotypic/genotypic correlations. It is likely to have a major role in the assessment of future treatments of retinal dystrophies, possibly involving gene therapy and photoreceptor rescue.
The authors practice at Moorfields Eye Hospital. Contact Mr. Hykin at: Moorfields Eye Hospital, City Rd., London EC1V 2PD, UK. Phone: 0044 20 7566 2057, fax: 0044 20 566 2608, e-mail: Philip.hykin@moorfields.nhs.uk.
1. Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Invest Ophthalmol Vis Sci 1978;17:583-600.
2. Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescence granules in cultured human RPE. Invest Ophthalmol Vis Sci 1989;30:82-89.
3. Katz ML, Gao CL, Rice LM. Formation of lipofuscin-like fluorophores by reaction of retinal with photoreceptor outer segments and liposomes. Mech Ageing Dev 1996;92:159-174.
4. Katz ML, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2001;42:3023-3030.
5. Delori FC. Spectrophotometer for noninvasive measurement of intrinsic fluorescence and reflectance of the ocular fundus. Appl Optics 1994;33:7439-7452.
6. Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995;36:718-729.
7. von Rückmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol 1995;79:407-412.
8. von Rückmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci 1997;38:478-486.
9. von Rückmann A, Fitzke FW, Bird AC. Distribution of pigment epithelium autofluorescence in retinal disease state recorded in vivo and its change over time. Graefes Arch Clin Exp Ophthalmol 1999;237:1-9.
10. Robson AG, El-Amir A, Bailey C, Egan CA, Fitzke FW, Webster AR, Bird AC, Holder GE. Pattern ERG correlates of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 2003;44:3544-3550.
11. Lois N, Halfyard AS, Bird AC, Fitzke FW. Quantitative evaluation of fundus autofluorescence imaged "in vivo" in eyes with retinal disease. Br J Ophthalmol 2000;84:741-745.
12. Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1978;17:601-607.
13. Feeney-Burns L, Hildebrand ES, Eldridge S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci 1984;25:195-200.
14. Weiter JJ, Delori FC, Wing GL, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci 1986;27:145-152.
15. von Rückmann A, Schmidt KG, Fitzke FW, Bird AC, Jacobi KW. [Fundus autofluorescence in patients with hereditary macular dystrophies, malattia leventinese, familial dominant and aged-related drusen]. [Article in German]. Klin Monatsbl Augenheilkd 1998;213:81-86.
16. Lois N, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol 2002;133:341-349.
17. Shiraki K, Kohno T, Moriwaki M, Yanagihara N. Fundus autofluorescence in patients with pseudoxanthoma elasticum. Int Ophthalmol 2001;24:243-248.
18. Scholl HP, Bellmann C, Dandekar SS, Bird AC, Fitzke FW. Photopic and scotopic fine matrix mapping of retinal areas of increased fundus autofluorescence in patients with age-related maculopathy. Invest Ophthalmol Vis Sci 2004;45:574-83.
19. Solbach U, Keilhauer C, Knabben H, Wolf S. Imaging of retinal autofluorescence in patients with age-related macular degeneration. Retina 1997;17:385-389.
20. Holz FG, Bellmann C, Margaritidis, Otto TP, Völcker HE. Pattern of increased in vivo fundus autofluorescence in the junctional zone of geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 1999;237:145-152.
21. Bellmann C, Jorzik J, Spital G, Unnebrink K, Pauleikhoff D, Holz FG. Symmetry of bilateral lesions in geographic atrophy in patients with age-related macular degeneration. Arch Ophthalmol 2002;120:579-584.
22. Holz FG, Bellman C, Staudt S, Schutt F, Volcker HE. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 2001;42:1051-1056.
23. Schmitz-Valckenberg S, Jorzik J, Unnebrink K, Holz FG;FAM Study Group. Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2002;240:73-78.
24. Robson AG, Egan CA, Luong VA, Bird AC, Holder GE, Fitzke FW. Comparison of fundus autofluorescence with photopic and scotopic fine-matrix mapping in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 2004;45:4119-4125.
25. Scholl HP, Chong NH, Robson AG, Holder GE, Moore AT, Bird AC. Fundus autofluorescence in patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 2004;45:2747-2752.
26. Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 2004;111:1585-1594.
27. Delori FC, Staurenghi G, Arend O, Dorey K, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt's disease—Fundus flavimaculatus. Invest Ophthalmol Vis Sci 1995;36:2327-2331.
28. von Rückmann A, Fitzke FW, Bird AC. In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol 1997;115:609-615
29. Jarc-Vidmar M, Kraut A, Hawlina M. Fundus autofluorescence imaging in Best"s vitelliform dystrophy. Klin Monatsbl Augenheilkd 2003;220:861-867.
30. Bellmann C, Neveu MM, Scholl HP, Hogg CR, Rath PP, Jenkins S, Bird AC, Holder GE. Localized retinal electrophysiological and fundus autofluorescence imaging abnormalities in maternal inherited diabetes and deafness. Invest Ophthalmol Vis Sci 2004;45:2355-2360.
31. Lois N, Holder GE, Bunce C, Fitzke F, Bird AC. Phenotypic subtypes of Stargardt macular dystrophy-fundus flavimaculatus. Arch Ophthalmol 2001;119:359-369.
32. von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol 2002;133:780-786.
33. Framme C, Sachs HG, Gabler B, Roider J. Fundus autofluorescence in APMPPE in association with lyme disease. Retina 2002;22:653-657.