The causes of uveitis vary widely depending upon geographic, cultural, environmental and socioeconomic factors, and upon the regional prevalence of causative agents within the environment.1 In many parts of the world, parasitic infections of the eye are a major cause of blindness. Diffuse unilateral subacute neuroretinitis (DUSN) is an ocular parasitic infection that usually results in severe visual loss.2
Diffuse unilateral subacute neuroretinitis has been associated clinically with two different-sized, and as of yet unidentified, nematodes, both of which appear to be capable of infiltrating the subretinal space.3-6 The term DUSN was first used by J. Donald M. Gass, MD, in 1978. Prior to that, the condition was referred to as "unilateral wipe-out syndrome."7 DUSN occurs in healthy patients, mainly in children and young adults, and the clinical course is characterized by periods of activity and remission. The intraocular inflammation tends to be unilateral and diffuse, and to be characterized in its early stages by vision loss, vitritis, papillitis and clusters of multiple evanescent, gray-white outer retinal lesions. In the later stages, optic atrophy, retinal artery narrowing, diffuse pigment epithelial alterations and an abnormal electroretinogram can be seen.8
Epidemiology and Causal Agent
Although DUSN initially was described in the southeastern and midwestern United States and the Caribbean islands, it since has been reported in other parts of North America, in South America and in northwestern Europe.8,9 In fact, in Brazil and other parts of South America, DUSN increasingly is considered an important cause of posterior uveitis in children and young healthy adults.10,11
|
Nevertheless, Arnaldo P. Cialdini, MD, and associates reported the first South American case of DUSN caused by a larger nematode.10 In earlier reports, serologic testing was negative in most patients with viable intraretinal nematodes, which led Dr. Gass and Robert Braunstein, MD, to suggest that Toxocara was not the causative nematode in most patients with DUSN,3 but rather that it was the dog hookworm Ancylostoma caninum, which is less than 1,000 µm in length. Kevin Kazacos, DVM, PhD, and colleagues, in contrast, suggested that the larger nematodes isolated from patients in the north midwestern United States was the raccoon ascarid, Baylisascaris procyonis.14
Because none of the nematodes described from patients with DUSN has ever been recovered intact, identification has been based upon a combination of careful measurement of the parasite, serologic testing and epidemiological studies, all of which have had their limitations.4 Such uncertainties have allowed some to suggest, based on morphology alone, that tropical varieties of T. canis and A. caninum may account for some cases of DUSN in Brazil and South America (Eduardo Cunha de Souza and associates, personal communication).
Clinical Features and Diagnosis
Recognition of DUSN early in the course of disease is extremely important, since removal or destruction of the worm may prevent further vision loss. Unfortunately, however, most patients seek treatment only after extensive damage has already been done, during late stages of the disease.
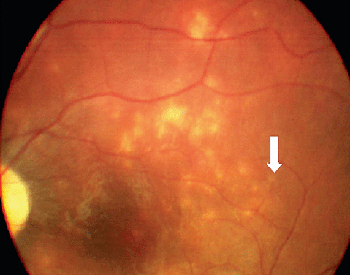
Because serologic testing has been variable, the diagnosis depends upon identification of characteristic clinical findings in conjunction with an intraocular worm.15 Clinical characteristics are manifested in early and late stages. DUSN most frequently is seen in healthy children or young adults with no significant past ocular history. The onset is frequently insidious. Some patients in the early stages of the disease complain of unilateral paracentral or central scotoma, ocular discomfort or transient obscuration of vision.5,7 The syndrome is characterized mainly by unilateral vitreous inflammation, optic disc swelling, and the presence of gray-white lesions in the deep retina.7,11 Early signs of DUSN often are mistaken for multifocal choroiditis, acute posterior multifocal placoid pigment epitheliopathy, multiple evanescent white dot syndrome, or nonspecific optic neuritis and papillitis (See Figure 2).
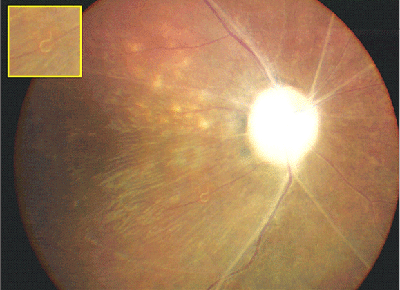
Late features of the disease include narrowing of the retinal vessels, optic nerve atrophy, and the development of focal or diffuse atrophic changes in the retinal pigment epithelium (See Figure 1).16 Although most patients with DUSN do not develop the disease in the fellow eye, bilateral DUSN has been described by Dr. de Souza and associates from Brazil.6
|
Ancillary Tests
Systemic evaluation, serologic testing, stool examinations and peripheral blood smears are of little value in making the diagnosis of DUSN. When a worm is identified within the eye of an otherwise healthy person, unless a peripheral eosinophilia is present, no further evaluation seems warranted to make the diagnosis.12
Most patients with DUSN have an abnormal electroretinogram, even if tested early in the course of the disease. Electroretinogram is rarely extinguished completely, however, which can help differentiate DUSN from the tapetoretinal degenerations.11,17 In one patient, multifocal electroretinography performed before laser treatment to destroy the worm showed decreased foveal response density, and increased parafoveal and perifoveal waveform amplitudes. Two months after laser photocoagulation of the nematode, multifocal electroretinography showed full recovery of normal findings, and visual acuity remained 20/20.18 Recently, it has been noticed that Goldmann perimetry may be useful to evaluate remaining visual field before and after treatment of the disease (Eduardo Cunha de Souza, personal communication, 2001).
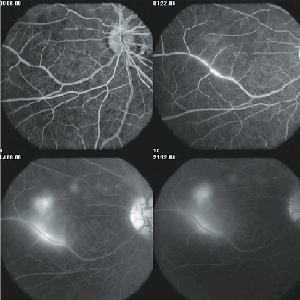
In the early stages of disease, fluorescein angiography can show hypofluorescence of the focal gray-white lesions of active retinitis followed by late staining. Leakage of dye is also typically seen from the capillaries on the optic disc. Occasionally, there is evidence of prominent perivenous leakage of dye (See Figure 3).
In more advanced stages of the disease, angiography shows greater evidence of loss of pigment from the RPE, manifested angiographically as an irregular increase in the background choroidal fluorescence.12 Indocyanine green angiography (ICG-A) suggests that the choroid is also involved in early-stage DUSN. Choroidal infiltration, which prevents normal choroidal ICG impregnation, most probably is the physiopathogenic explanation for the hypofluorescent dark spots seen in affected eyes. The dark spots present in the initial ICG-A phase seem to either disappear or persist in the late phase of the examination. Hypofluorescent dots persisting in the late phase are interpreted as full-thickness lesions allowing no ICG diffusion; dots becoming isofluorescent in the late phase are interpreted as partial-thickness lesions progressively surrounded by ICG fluorescence.19
|
Differential Diagnosis
Early signs of DUSN often are mistaken for those of sarcoidosis and other entities that cause focal chorioretinitis including toxoplasmosis and histoplasmosis, multifocal choroiditis, serpiginous choroiditis, acute posterior multifocal placoid pigment epitheliopathy, multiple evanescent white dot syndrome, nonspecific optic neuritis and papillitis. The late stage of DUSN is often mistaken for post-traumatic chorioretinopathy, occlusive vascular disease, sarcoidosis or toxic retinopathy.12,16
Therapy
Treatment is limited in patients with DUSN. At present, treatment of a visible worm with photocoagulation seems to offer the best chance for halting worm motility and resolution of the active gray-white lesions without causing significant intraocular inflammation or toxic damage to the eye. Some improvement in vision and visual field may occur after laser treatment of the worm.20 In some patients with the worm very close to the center of the fovea in which heavy photocoagulation may damage the remaining central vision, it may be possible to use very light applications of the laser to chase the worm into the mid-periphery, where it may be destroyed with less retinal damage.16
Chemotherapy with anthelmintic drugs, such as thiabendazole, may be the only treatment available when a worm cannot be visualized, but success is often difficult to document. Dr. de Souza and colleagues described 12 patients whose VA, visual field and intraocular inflammatory signs improved following treatment exclusively with high-dose oral albendazole (400 mg/d) for 30 days.21 Treatment with corticosteroids has shown transient suppression of the inflammation without altering the final outcome of the disease.16
Pars plana vitrectomy is not the standard of treatment for DUSN whenever laser is possible. At times, however, vitrectomy may be favorably compared to photocoagulation.16
In summary, DUSN is a potentially blinding form of uveitis caused by ocular nematode infestation. The diagnosis should be suspected in children and young adults with unilateral uveitis associated with multifocal choroiditis, disproportionate vision loss given the clinical findings, and little or transient response to corticosteroids. Definitive treatment involves destruction or removal of the nematode.
Dr. Sabrosa is director of the Medical Retina and Vitreoretinal Services at Santa Casa de Misericordia do Rio de Janeiro (1a Enfermaria), and is in private practice at Clínica São Vicente. Dr. Arevalo is director of the Clínica Oftalmológica Centro Caracas and director of the Retina and Vitreous Service, Edif. Centro Caracas PH-1, Av. Panteón, San Bernardino, Caracas, 1010, Venezuela. Dr. Sabrosa may be contacted at Clínica de Olhos Gávea, Rua João Borges, 204, Gávea, Rio de Janeiro, Brazil, CEP 22451-100. Phone and fax: (+55-21) 2259-5046; e-mail:
nsabrosa@terra.com.br.
1. Rao NA, Forster DJ. General approach to the uveitis patient. In: Podos JM, Yanoff M, editors. Textbook of ophthalmology. New York: Gower Medical Publishing, 1992.
2. Rathinam SR, Cunningham ET Jr. Infectious causes of uveitis in the developing world. Int Ophthalmol Clin 2000;40:137-152.
3. Gass JD, Braunstein RA. Further observations concerning the diffuse unilateral sub acute neuroretinitis syndrome. Arch Ophthalmol 1983;101:1689-1697.
4. Goldberg MA, Kazacos KR, Boyce WM, et al. Diffuse unilateral sub acute neuroretinitis. Morphometric, serologic, and epidemiologic support for Baylisascaris as a causative agent. Ophthalmology 1993;100:1695-1701.
5. Gass JDM. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, 3rd ed. Vol.2. St. Louis: CV Mosby, 1997:622-628.
6. de Souza EC, Abujamra S, Nakashima Y, et al. Diffuse bilateral sub acute neuroret-initis: First patient with documented nematodes in both eyes. Arch Ophthalmol 1999;117:1349-1351.
7. Gass JD, Gilbert WR Jr, Guerry RK, et al. Diffuse unilateral sub acute neuroretinitis. Ophthalmology 1978;85:521-545.
8. Harto MA, Rodrigues-Salvador V, Avino JA, et al. Diffuse unilateral sub acute neuroretinitis in Europe. Eur J Ophthalmol 1999;9:58-62.
9. Casella AMB, Bonomo PP, Farah ME, et al. Diffuse unilateral sub acute neuroretinitis (DUSN): Three cases in Parana State. Arq Bras Oftalmol 1994;57:77-79.
10. Cialdini AP, de Souza EC, Avila MP. The first South American case of diffuse unilateral sub acute neuroretinitis caused by a large nematode. Arch Ophthalmol 1999;117:1431-1432.
11. Sabrosa NA, de Souza EC. Nematode infections of the eye: Toxocariasis and diffuse unilateral subacute neuroretinitis. Curr Opin Ophthalmol 2001;12:450-454.
12. Garcia CA, Sabrosa NA, Gomes AB, et al. Diffuse unilateral subacute neuriretinitis–DUSN. Int Ophthalmol Clin 2008;48(3):119-129.
13. Gass JDM, Olsen KR. Diffuse Unilateral Sub acute Neuroretinitis. In: Ryan SJ, Schachat AP, eds. Retina, Third edition. St. Louis: CV Mosby, 2001:1669-1678.
14. Kazacos KR, Raymond LA, Kazacos EA, et al. The raccoon ascarid.A probable cause of human ocular larva migrans. Ophthalmology 1985;92:1735-1744.
15. Garcia CA, Gomes AH, Vianna RN, et al. Late-stage diffuse unilateral subacute neuroretinitis: Photocoagulation of the worm does not improve the visual acuity of affected patients. Int Ophthalmol. 2005;26:39-42.
16. Matsumoto BT, Adelberg DA, Del Priore LV. Transretinal membrane formation in diffuse unilateral subacute neuroretinitis. Retina 1995;15:146-149.
17. Davis JL, Gass DM. Diffuse unilateral sub acute neuroretinitis. In: Pepose JS, Holland GN, Wilheumus KR, editors. Ocular infection and immunity. St. Louis: Mosby-Year Book;1996:1243-1247.
18. Martidis A, Greenberg PB, Rogers AH, et al. Multifocal electroretinography re-sponse after laser photocoagulation of a subretinal nematode. Am J Ophthalmol 2002;133:417-419.
19. Vianna RN, Onofre G, Ecard V, et al. Indocyanine green angiography in diffuse unilateral sub acute neuroretinitis. Eye 2006;20:1113-1116.
20. Garcia CA, Gomes AH, Garcia Filho CA, et al. Early-stage diffuse unilateral sub acute neuroretinitis: Improvement of vision after photocoagulation of the worm. Eye 2004;18:624-627.
21. de Souza EC, Casella AM, Nakashima Y, et al. Clinical features and outcomes of patients with diffuse unilateral sub acute neuroretinitis treated with oral albendazole. Am J Ophthalmol 2005;140:437-445.