The incidence of dry eye has been on the rise in recent years, and the pandemic has furthered the problem. “Screen time is increased when people are working from home and are on computers all day, as opposed to being in an office where they are walking around, talking to people and going to meetings,” says Christopher J. Rapuano, MD, chief of Wills Eye Hospital’s Cornea Service. “It’s well-known that screen use increases dry-eye symptoms. Masks can also contribute to dry eye for a lot of people.”
Fortunately, researchers are looking at new ways to treat the condition. Here’s a look at some of the drugs and devices in the pipeline.
Reproxalap
 |
|
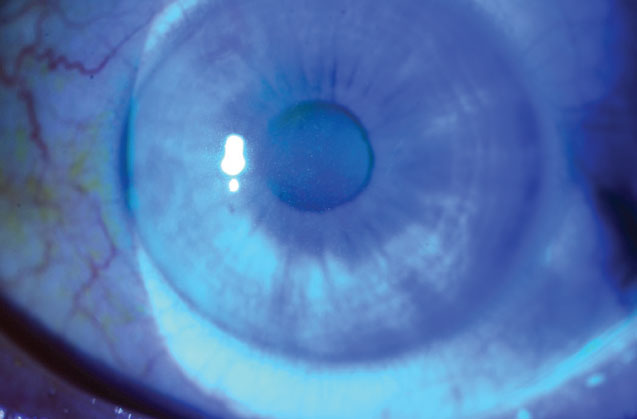
Mild diffuse superficial punctate keratopathy in a patient with moderate dry-eye syndrome. Image courtesy of Christopher J. Rapuano, MD. |
Reproxalap (Aldeyra), a promising new agent, is a small-molecule reactive aldehyde species (RASP) inhibitor that covalently binds free aldehydes and diminishes excessive RASP levels.
In a recent study, reproxalap demonstrated rapid, broad and clinically relevant symptomatic control in dry-eye patients over 12 weeks of therapy.1 Additionally, there was statistically significant improvement compared to vehicle in signs of dry-eye disease, as demonstrated by fluorescein staining. The results represent the first vehicle-controlled evidence for the therapeutic potential of RASP inhibition to ameliorate the signs and symptoms of dry-eye disease.
“RASP inhibition provides a novel mechanism of action that appears to have steroid-like efficacy and tolerability, without steroid side effects,” notes John Sheppard, MD, practicing with Virginia Eye Consultants and CVP Partners, and serving as professor of ophthalmology at Eastern Virginia Medical School in Norfolk.
RGN-259
RGN-259 (RegeneRx) is a Tβ4-based sterile and preservative-free eye drop that’s designed to be a novel treatment for dry eye and neurotrophic keratitis. Recently, the ARISE-3 Phase III clinical trial evaluating RGN-259 eye drops for the treatment of dry eye didn’t meet its primary outcome measures, according to the company. However, researchers noted statistically significant improvement in ocular grittiness at one and two weeks after treatment, and post-exposure in a controlled adverse environment after two weeks of treatment with the drug, compared to placebo. Additionally, they say RGN-259 continued to demonstrate safety in the treatment of dry eye consistent with previous clinical trials.
Visomitin
Visomitin (Mitotech) is an eye-drop formulation of the drug SkQ1 that’s been developed to target ophthalmic disorders like dry eye, uveitis and macular degeneration.
The company says that SkQ1 was designed to address dry eye through a novel mechanism of action that consists of acting on the mitochondria at a cellular level. It belongs to the class of cardiolipin peroxidation inhibitors developed for the treatment of several age-related disorders, including dry eye. In contrast to current standards of care, which act primarily as anti-inflammatory agents, Mitotech says that SkQ1 has been shown to not only relieve inflammation but also to mitigate tissue degeneration and improve tear quality deficit by targeting oxidative stress within the eye. VISTA-1, a Phase IIb/III clinical study in the United States (NCT03764735), found that SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in dry-eye subjects.
Mitotech and Essex Bio-Technology recently announced completion of enrollment in a pivotal Phase III VISTA-2 study of SkQ1 ophthalmic solution in patients with moderate to severe dry eye.
VISTA-2 is a multicenter, randomized, double-blind, placebo-controlled clinical study with two treatment arms, one receiving SkQ1 and one receiving vehicle administered twice daily. The study includes 610 patients in multiple centers across the United States who will receive treatment over a two-month period.
VISTA-2 was designed to confirm the outcome of VISTA-1.
OC-01
OC-01 (Oyster Point Pharmaceuticals) is a highly selective nicotinic acetylcholine receptor (nAChR) agonist being developed as a preservative-free nasal spray to treat the signs and symptoms of dry eye. The company says the drug’s novel mechanism of action activates the trigeminal parasympathetic pathway in the nasal cavity to stimulate natural tear-film production.
Oyster Point says the Phase III ONSET-2 clinical trial yielded positive top-line results. This multicenter, randomized, double-masked, vehicle-controlled clinical trial included 758 subjects at 22 centers in the United States and investigated two doses of OC-01 nasal spray (0.6 mg/mL and 1.2 mg/mL), as compared to control (vehicle) nasal spray. Subjects were administered OC-01 nasal spray b.i.d. for four weeks.
Both tested doses of OC-01 showed a statistically significant improvement, with subjects gaining 10 mm or more in Schirmer’s score at week four when compared to control. The percentage of patients gaining 10 mm or more on the Schirmer’s test was 44 percent in the 0.6 mg/mL dose group, 47 percent in the 1.2 mg/mL dose group, and 26 percent in the control group.
Additionally, there was a statistically significant improvement in mean change in Schirmer’s score at week four in both doses tested when compared to control.
Stephen Pflugfelder, MD, from the Baylor College of Medicine in Houston, believes that this product may be the next one to reach the market. “It’s shown significantly greater increase in its endpoint of increased tear production,” he says. “It offers a different approach, where it can provide an on-demand increase in tear production by using the nasal spray.”
He says that he’s excited about this drug, which appears to have a good safety profile. “It’s the same medicine that’s used in Chantix, which is a medicine people take to stop smoking,” he says. “Oyster Point discovered that this drug can stimulate receptors in the nasal cavity and increase tear production. So, if the drug has the same efficacy as in the clinical trial, that’s probably the drug I’m most excited about because it’s a different mechanism of action and a different approach.”
Nov03
NOV03 (Bausch + Lomb) is an investigational, proprietary, water-free and preservative-free solution, based on patented EyeSol technology from Novaliq GmbH.
The GOBI trial is the first Phase III trial evaluating the investigational drug NOV03 (perfluorohexyloctane) as a first-in-class eye drop with a novel mechanism of action to treat the signs and symptoms of dry-eye disease associated with meibomian gland dysfunction.
The GOBI trial included results from 597 participants aged 18 years and older who were randomized to receive either treatment with NOV03 or administration of placebo four times daily. The multicenter, randomized, double-masked, saline-controlled Phase III study was conducted at 26 locations in the United States.
NOV03 was well-tolerated, with the incidence of instillation site reactions below 0.5 percent. No treatment-emergent adverse events were reported by more than 2 percent of subjects in either treatment group.
“This product will most certainly make it to market,” Dr. Sheppard opines. “It has a unique vehicle that’s water-free, so it doesn’t need a preservative. It’s not metabolized, so any excess simply drains into the nasolacrimal system without systemic absorption. Because of its polymeric molecular nature, the drop is 20-µL instead of 50 like a regular solution or suspension drop. The cul-de-sac tear lake will only hold about 20 to 30 µL of volume, so its 20-µL volume is perfectly suited to the volume of the ocular surface. As a long-chain semifluorinated alkane, it has a direct stabilization effect on the meibomian glands, and then it solubilizes the meibomian lipid secretions from the outside in and has an inherent anti-inflammatory effect.”
CyclASol
CyclASol is a topical anti-inflammatory and immunomodulating ophthalmic solution, containing 0.1% cyclosporine A in EyeSol, developed for the treatment of dry-eye disease. The unique water-free drug product is based on the EyeSol enhanced ocular bioavailability technology that allows for several-fold higher corneal penetration of cyclosporine A in comparison to water or oil-based formulations. The previous Phase IIb/III trial (ESSENCE-1) evaluated the efficacy, safety, and tolerability of CyclASol in patients with dry eye.2 In that study, CyclASol demonstrated statistically significant improvements in pre-specified endpoints for both signs and symptoms of dry eye when compared to its vehicle after four weeks.
The ongoing ESSENCE-2 trial is a multicenter, randomized, double-masked, vehicle-controlled clinical trial assessing efficacy, safety and tolerability of CyclASol for the treatment of signs and symptoms of dry eye. Positive results from ESSENCE-2 will allow for a New Drug Application filing to the US FDA in 2022, according to the company.
“The folks at Novaliq have shown that this shorter-chain semi-fluorinated alkane vehicle produces an excellent solubilization of the medication and allows it to remain on the ocular surface for an extended period of time to optimize absorption into the ocular surface tissues and the lacrimal gland,” Dr. Sheppard says. “Again, the outstanding feature of this particular vehicle is that it’s extremely well-tolerated. The self-discontinuation rate is well below 3 percent, and the side effects of stinging, burning and blurring are essentially equivalent to vehicle. This [patient comfort] sets it apart from other preparations—at least in the trials. The real world, though, is always full of surprises.”
Lacripep
Lacripep (Tear Solutions ) is a first-in-class topical synthetic peptide treatment for dry eye. It’s a synthetic tear protein fragment of Lacritin, which is a nanomolar concentration constituent of normal human tears, but is lacking in the tears of dry-eye patients.
Preliminary outcomes of a Phase I/II trial of Lacripep in primary Sjögren’s syndrome patients were recently released. The trial was designed to test proof-of-concept and optimize the design for the next planned study. Two strengths of Lacripep were tested. The lower-strength agent exhibited a highly statistically significant reduction in inferior corneal staining, as well as a statistically significant improvement in burning and stinging.
“This biologic medication accurately molecularly mimics Lacritin,” Dr. Sheppard notes. “Lacritin is present in all eyes, but it’s decreased in dry eye. Because of proof-of-concept in a meticulous Phase II trial, we’re encouraged that Lacripep will have much broader applicability for less-severe dry eye or less-advanced surface disease.”
EFC843
Novartis is studying a biologic lubricant found in synovial fluid, ECF843. The agent is a recombinant human lubricin (boundary lubricant) and an investigational compound. Efficacy and safety of ECF843 have yet to be established.
HBM9036
Tanfanercept (HBM9036, Harbour BioMed) is a modified 19-kDa TNF receptor 1 fragment. The drug is molecularly engineered as a therapy for relief of the signs and symptoms of dry eye. It was specifically developed for ophthalmic topical use with good surface permeability, strong TNF-α neutralizing activity, high stability and minor side effects, according to Harbour BioMed.
AR-15512
Aerie’s TRPM8 agonist recently completed enrollment of its Phase IIb clinical trial. The company says that, when activated, the TRPM8 receptor may increase tear production and produce a cooling sensation to decrease discomfort.
AZR-MD-001
This drug, from Azura Ophthalmics, is in Phase II trials for the treatment of MGD. Azura says the new agent met its primary endpoints, showing improvements in signs and symptoms of MGD, reaching statistical significance when compared to controls.
TP-03
Tarsus’ TP-03 is currently in Phase IIb/III trials for Demodex blepharitis, but is also in early trials as a dry-eye treatment. The company says the agent is designed to paralyze and eradicate mites and other parasites, and was well-tolerated in initial trials.
SURF-200
Surface Ophthalmics’ agent recently entered Phase II trials for acute dry eye. SURF-200 is 0.2% betamethasone in the company’s Klarity vehicle. It’ll be studied in two different low-concentration formulations in 120 to 140 patients.
Though the dry-eye field is already filled with options for patients and their physicians, clinicians are always willing to give a new method a try. “Many patients just don’t do well with the current treatments for dry eye,” Dr. Rapuano says, “so it’s exciting to see so many new products in development.”
Dr. Rapuano is a consultant for TearLab and Oyster Point. Dr. Pflugfelder is a consultant for Oyster Point. Dr. Sheppard is a consultant for Abbvie, Allergan, Aldeyra, Bausch & Lomb, LacriSciences, Mitotech, Novartis, Novaliq, Oyster Point, Quidel, Sun Pharma and TearLab. He has equity interest in LacriSciences, Oyster Point and TearLab.
1. Clark D, Tauber J, Sheppard J, Brady TC. Early onset and broad activity of reproxalap in a randomized, double-masked, vehicle-controlled phase IIb trial in dry eye disease. Am J Ophthalmol 2021;226:22-31.
2. Sheppard, JD, Wirta DL, McLaurin E, et al. A water-free 0.1% cyclosporine A solution for treatment of dry eye disease. Results of the randomized Phase 2B/3 ESSENCE study. Cornea 2021. doi: 10.1097/ICO.0000000000002633. Epub ahead of print. Accessed Feb. 1, 2021.