In recent years, time-domain optical coherence tomography has attained wide acceptance as a tool for glaucoma diagnosis, even though its images of the optic nerve head and nerve fiber layer are not always as detailed as those produced by other imaging technologies. Because of its intrinsically higher axial resolution, this technology has always had the potential for evolving and producing significantly better images. That promise has now begun to be realized with the development of spectral-domain OCT (also known as Fourier-domain OCT and high-definition OCT).
The improvement in image quality is largely the result of dramatically increased scanning speed. This has improved the signal-to-noise and ratio sampling rate, and that, combined with an even higher axial resolution, makes it possible to obtain a much more detailed image of the optic nerve head and the surrounding retinal nerve fiber layer. The greater speed also reduces the variability caused by motion artifact that accompanies slower image acquisition. In terms of glaucoma, this improvement translates to the potential for a much more detailed look at how the disease affects the optic nerve and RNFL, which should make it easier to distinguish between glaucomatous and normal eyes, and make it easier to identify glaucoma progression.
That's the potential. The question is: Are we there yet?
Vive La Différence
To appreciate the difference between time-domain OCT data and SD-OCT data, it's important to understand that when TD-OCT is used for nerve fiber layer thickness measurements, all of the diagnostic information is taken from a single circumpapillary scan—a circular cross-sectional image of the retina around the optic nerve head. Similarly, if you use the instrument for imaging the optic nerve head, it takes six cross-sectional cuts across the optic nerve and then interpolates the topography of the nerve head between these individual slices of data. In short, very little information is actually available or used. The reason for this is the slow scanning speed; eye motion interferes if you try to obtain more information.
In contrast, SD-OCT technology can quickly image the entire optic nerve head and peripapillary retina. You get nerve fiber layer thickness information from the entire region surrounding the optic nerve rather than from a single slice, and topographic information from the entire optic nerve head. Combined with the increased resolution of every scan, much more information is acquired, allowing these instruments to provide images and information far superior to that provided by time-domain OCT instruments. For example, an SD-OCT scan can show you the papillary retinal thickness in a particular location of your choice, and a qualitative evaluation of images obtained with this technology has already demonstrated that it's possible to visualize structures that you can't visualize with time-domain OCT.1
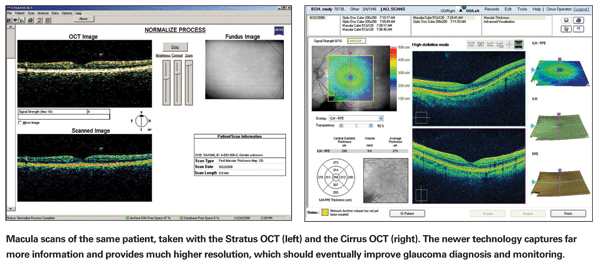
This kind of improved image quality and data acquisition has already made SD-OCT very useful for retinal specialists treating macular disease. SD-OCT can quantify the thickness of specific layers of the retina more accurately than TD-OCT; it makes it possible to identify problems in the internal structure of the retina far more easily. But for a glaucoma specialist, a more detailed view and more accurate measurements aren't always as helpful; they don't necessarily tell us how an individual patient's measurements compare to other scans of normal individuals and glaucoma patients, or to previous scans of the same patient. To eliminate subjectivity, we need to have something more than just our own clinical impression on which to base a diagnosis. A statistical, comparative database provides an objective, repeatable method of analysis that's independent of the observer and not prone to human error, and thus allows us to have confidence about the conclusions we're drawing.
Bridging the Software Gap
The problem is that we don't yet have comparative data based on the kind of detailed information SD-OCT can provide. We need that information, incorporated into software, to really take advantage of the possibilities of SD-OCT in glaucoma. Currently, multiple SD-OCT instruments are commercially available—and diagnosing and monitoring glaucoma is one of the major applications of OCT technology that manufacturers hope to address. Nevertheless, the software for identifying glaucoma and performing progression analysis has been very limited to date. It hasn't yet taken full advantage of the potential of this new technology.
All of these devices need a database of normative information so that individual results can be compared to population norms, to help distinguish glaucomatous eyes from normals. So far, only Zeiss' Cirrus HD-OCT has incorporated such a database, and it's limited to distinguishing points in the nerve fiber layer thickness measurement map that are borderline or outside the expected normal range. There's no statistical guidance as to how large or deep a defect needs to be before it's really abnormal. As a result, the final interpretation of the image remains quite subjective, except in cases where the glaucoma is more advanced, making the diagnosis fairly straightforward. [Note: As this article went to press, Optovue announced the release of new software for the Optovue RTVue, including a normative glaucoma database.]
These instruments also need studies of variability over the course of repeat images in order to be able to develop progression software. These will allow the user to determine the likelihood that abnormalities in a scan truly represent developing glaucoma, and whether differences between scans in an individual patient represent a real change or fall within the expected range of variability based on the characteristics of that instrument.
The reality is, until that software becomes available, the qualitative improvement of the images isn't going to add much to our capabilities in terms of glaucoma diagnosis. We may note that the optic nerve looks a little abnormal; in some cases, we may feel pretty sure about our diagnosis. In other cases we may even be positive, but the reality is that our conclusion is subjective. Without that comparative software database, we're almost back where we were before optic nerve imaging became available.
Zeiss' Cirrus HD-OCT, as well as some of the other commercially available SD-OCT instruments, includes software for glaucoma that gives the same sort of report as their earlier time-domain Stratus OCT. However, for the sake of making the glaucoma analysis comparable to what people are accustomed to, this part of the Cirrus analysis only considers data taken from a circular ring around the optic nerve head, just as the Stratus does. It ignores all the extra data that the SD-OCT instrument is capable of obtaining. Using the software in this way allows continuity, but it doesn't take full advantage of what the new instrument can do. (The Cirrus analysis does provide a nerve fiber layer thickness map of the peripapillary retina, but it still requires a subjective decision about how abnormal the result must be to represent disease.)
I suspect manufacturers are making their new scans resemble the older ones for several reasons. First, it makes the new instrument easily usable by doctors familiar with the earlier format, even though the result doesn't use the new data to full advantage. Second, it gets the instrument on the market with at least some glaucoma software. Third, it makes it possible for doctors to bill when they use the instrument for this purpose.
Should You Buy Today?
Many ophthalmologists with an interest in glaucoma are wondering whether it makes sense to invest in one of the SD-OCT instruments at this point in time. The answer is ... it depends.
I've worked with several of the different SD-OCT instruments at the Cleveland Clinic as part of a comparative study. It's not yet clear to me which of the devices performs best from a hardware standpoint. From a software standpoint, I think the Cirrus OCT is probably the most developed, but it's far short of what it could be, given the capabilities of the instrument. Still, that could change at a moment's notice. We frequently receive software updates from each of the companies, and it's only a matter of time before the next generation of glaucoma software applications becomes available.
One important consideration is that there are other uses for SD-OCT technology; the instrument has broad applications in the treatment of retinal disease (macular disease in particular). If these capabilities would be of value in your practice, that may be a reason to get into the market now. If you don't have that urgent need, then it might make sense to wait a little while for it to become clearer which instrument is most useful for glaucoma diagnosis.
Another consideration is that as software becomes available (hopefully in the near future) it should be possible to upgrade your device to take advantage of its glaucoma potential. Naturally, this is something you need to address with whatever salesperson you're talking to about your purchase. Make sure the manufacturer of the instrument is working on this and that you'll be able to implement software upgrades for glaucoma as they become available.
Suppose you haven't yet purchased any OCT instrument. Should you invest in the more advanced SD-OCT technology? You can probably get a time-domain OCT at bargain prices, given the advent of the new instrument. My answer would be yes, invest in the newer technology; it's such a huge jump forward that it would be a shame to start off several years behind the times. SD-OCT is the technology of the future; time-domain OCT is on the way out.
On the other hand, you may not want to jump in at all right now. This technology has only recently been introduced. As with any new technology, the cost will come down over time because of competition; and which instrument has the best hardware and software will gradually work itself out. (Unlike time-domain OCT, which was proprietary with the patent rights held by a single company, SD-OCT is not held with the same level of patent rights, so a number of different companies are marketing these instruments.) Plus, the most useful glaucoma software probably doesn't exist yet. Under the circumstances, waiting a little longer to invest in this technology may make perfect sense for some ophthalmologists.
The Best Is Yet to Come
Because the image quality and amount of data obtained by SD-OCT is vastly superior to that which time-domain OCT can generate, there's no doubt in my mind that glaucoma diagnosis will be improved with this instrument. So far, that potential just hasn't been translated into an off-the-shelf software package that's been validated, and that will be key to taking advantage of this technology's potential. But I'm confident that it will happen soon.
Dr. Smith is currently the director of the glaucoma service at
1. Mumcuoglu T, Wollstein G, Wojtkowski M, Kagemann L, Ishikawa H, Gabriele ML, Srinivasan V, Fujimoto JG, Duker JS, Schuman JS. Improved visualization of glaucomatous retinal damage using high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology 2008;115:782-789.