The DRCR Network recently completed Protocol U, a short-term study comparing the addition of a dexamethasone implant (Ozurdex) to ranibizumab (Lucentis) in patients who have continued diabetic macular edema.1 The impetus for this study was to see whether combined treatment with a corticosteroid and an anti-VEGF agent would improve vision, since, on average, about 40 percent of patients with DME will continue to have retinal thickening as well as reduced vision despite regular anti-VEGF injections over six months. This result was shown in DRCR Protocol I (in the Lucentis groups), as well as in Protocol T (where aflibercept groups had even lower rates of residual edema).
In this article, I’ll explain how we DRCR network researchers designed Protocol U, and what its findings might mean for the clinician.
The Study’s Design
In Protocol U, we wanted to see whether adding a steroid implant every three months to eyes that continued to have edema would result in improved visual acuity and decreased macular edema over the next six months compared to Lucentis alone.
For the study, we enrolled 236 patients who had received at least three injections of any
 |
anti-VEGF over the prior 20 weeks, but who continued to have macular edema and visual loss. These subjects were all given additional injections of Lucentis every four weeks for 12 weeks (i.e., three additional doses). At the end of this enrollment period, subjects were evaluated to see whether they still had macular edema (over 300 µm on SD-OCT) and visual loss (worse than BCVA of 20/25 on a standardized ETDRS refraction). A total of 129 subjects who still had edema and vision loss were randomized to either receive a combination of Ozurdex and Lucentis or to continue with Lucentis alone. It’s important to note that among those who were not eligible for randomization, many had either resolution of macular edema or improvement of vision before randomization with three additional injections of Lucentis, again demonstrating the benefit of continued anti-VEGF treatment.
While patients had to have persistent edema despite at least three injections of Lucentis prior to enrollment, we found that providing these same eyes with three additional injections of Lucentis in the enrollment phase resulted in continued improvement in vision and edema. Their vision in the enrollment phase improved on average by three letters, and their edema was reduced by more than 50 µm on average. In fact, about a third of the enrolled subjects either showed resolution of macular edema or achieved vision better than 20/25, and thus were exited from the study. The remaining eligible 129 subjects were randomized per protocol and received an average of 5.6 injections of Lucentis over the next 24 weeks, with the combination group receiving nearly two dexamethasone implants, on average, over the study period as well. Thus, subjects had close to maximal treatment through the duration of the study.
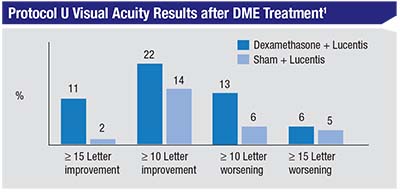
Results of the primary endpoint analysis were consistent with results from previous smaller single-center trials: We found no overall differences in visual acuity between the two groups (Lucentis alone vs. Lucentis + dexamethasone implant). Both groups improved by approximately three additional letters in the randomized, 24th-week phase of the study. However, on pre-planned subgroup analysis, we found that there was a greater proportion of subjects with a 15-letter or greater improvement in vision in the combination vs. the Lucentis-alone group (11 percent vs. 2 percent, p=0.03). We also found that the combination group had a small increase in the proportion of patients with a decrease in vision greater than 10 letters (13 percent vs. 6 percent, p=0.09), though this wasn’t statistically significant.
We also found that the central subfield thickness decreased significantly more in the combination group compared to the Lucentis-alone group (110 µm vs. 62 µm, p<0.001). Moreover, about half of the combination group had a flat retina at week 24
 |
compared to 31 percent of the Lucentis-alone group (p=0.02), and the improvement in OCT thickness was rapid and sustained in the combination group. This would be expected, as dexamethasone implants were provided every 12 weeks, and not every 24 weeks as per the label, since we felt that the duration of action of these implants required implantation at shorter intervals.
The entry criteria for the study were rather strict with regard to potential subjects not having any history of corticosteroid responsiveness. For example, subjects with a history of developing ocular hypertension in response to topical corticosteroids in the fellow eye at any time in their history were excluded. Despite this careful exclusion, about 30 percent of the combination group had a significant rise in intraocular pressure, with 20 percent of subjects in the combination group requiring treatment for increased IOP.
We found no visual acuity benefit in the combination group over the Lucentis-alone group six months after randomization. It’s possible that we would have had different results had the study been longer in duration. However, the visual benefits of adding steroids appear limited in this population. While there was a significant improvement in macular thickness in the combination group, this benefit must be balanced by the high incidence of IOP rise.
Subgroup Analyses
We did multiple subgroup analyses on these subjects:
• Pseudophakic patients. The study was initially conceived as a pseudophakic subject-only study. However, it was very difficult to recruit for the study, so we finally expanded the study to include phakic subjects. We examined the pseudophakic subgroup and found that the visual acuity tracked in line with the phakic subgroup acuity through week 20. At the end of the study (week 24 after randomization) the pseudophakic subgroup on combination treatment had a five-letter gain in acuity while the phakic subgroup treated with Lucentis alone had a gain of two letters. This difference at this single time point didn’t reach statistical significance.
• Duration of DME. Approximately half the subjects had only three injections of anti-VEGF prior to enrollment, and thus received three additional injections in the run-in phase. Therefore, many subjects in the combination group received only six total injections of anti-VEGF prior to receiving dexamethasone implants. This group’s visual acuity didn’t improve compared to subjects who had a longer duration of anti-VEGF prior to enrollment. Thus, in this study, earlier treatment with dexamethasone implants didn’t appear to result in improved acuity over anti-VEGF alone. Again, the relevance of this finding is limited by the small sample size.
Personal Experience
In my clinical practice, I use intraocular corticosteroids, mostly dexamethasone implants, in select cases. I’m most likely to use the implant in patients who continue to have mild edema despite the use of anti-VEGF therapy, particularly when they’re scheduled for cataract surgery in the near future. Pretreatment with a dexamethasone implant in these cases seems to prevent a worsening of macular edema that can occur following surgery. Otherwise, I believe there’s only a very limited role for corticosteroids in phakic patients.
There may be a benefit to having a retina without edema vs. a retina that continues to have edema, even if the measured ETDRS visual acuity is the same. The DRCR studies show that a patient can have stable and even improving vision in the presence of edema for at least two years. However, we may be missing some visual function that is useful for the patient, even if it’s not measurable with high contrast ETDRS testing. For example, a patient’s low-contrast acuity, useful in the dark, may be limited with increased edema. We just don’t know, as few studies look at such variables closely.
Despite the potential benefit of corticosteroids for reducing edema by about 50 percent more than anti-VEGF alone, the side-effect profile of intraocular corticosteroids makes them a less desirable choice. In phakic patients, the risk of cataracts is sufficiently high that I try to avoid their use. Other studies have shown comparable benefit to using steroids with anti-VEGF vs. steroids alone, finding that this approach may limit both the treatment and the cost burdens. However, before going this route, it’s best to confirm that the underlying diabetic retinopathy is well-controlled, as I believe corticosteroids don’t regress diabetic retinopathy as well as anti-VEGF agents do. In addition, while dexamethasone implant placement may require treatment only every 12 weeks, many patients will require intraocular pressure monitoring every four to eight weeks.
In summary, there are definite anatomic benefits to using Ozurdex over using Lucentis as studied in Protocol U. However, though overall acuity was similar in both groups in the study, the benefits of steroids, and therefore their usage, are limited by their side-effect profile. REVIEW
Dr. Maturi is an associate professor at the Indiana University School of Medicine. He receives grant support from Allergan and Genentech.
1. Maturi RK, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema. A DRCR Network phase II randomized clinical trial. JAMA Ophthalmol 2017; DOI:10.1001/jamaophthalmol.2017.4914.



