Guidelines for how to diagnose and characterize ocular surface disease have been available for years. Yet a 2019 survey by the American Society of Cataract and Refractive Surgery found that many surgeons don’t know the guidelines, even though they realize the disease can affect surgical outcomes.1
“I think most of us want to diagnose OSD accurately but, frankly, the disease is more complex than a lot of us realize,” says Kenneth Beckman, MD, a clinical assistant professor of ophthalmology at Ohio State University.
Performing cataract surgery on patients with unrecognized OSD can lead to refractive errors, OSD exacerbations and dissatisfaction with surgical outcomes.1,2 Significant lid destruction3 can occur in nonsurgical patients, and the disease can destroy more than half of the meibomian glands of otherwise healthy patients in their 20s.
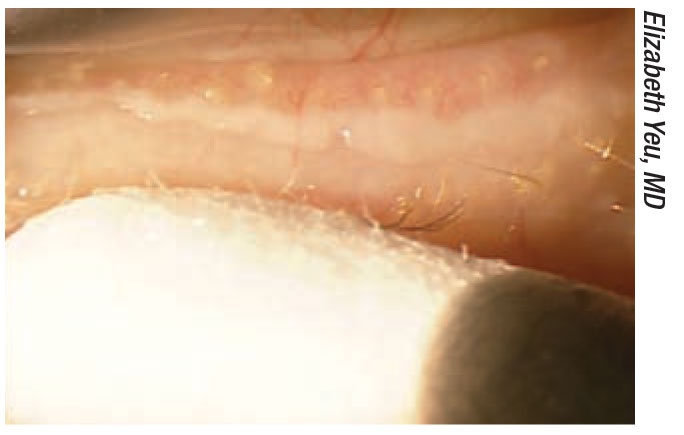 |
| Figure 1. Expressing meibum in patients with meibomian gland dysfunction can help remove bacteria and debris, as well as stimulate the glands. For a video showing Dr. Yeu performing meibomian gland expression, visit vimeo.com/355159648. |
OSD patients of all ages can develop photophobia, corneal scarring, intermittent blurred vision, pain, limited ability to perform daily activities, reduced vitality, poor general health and, in many cases, depression.4,5
Do you screen thoroughly enough to spare patients these problems? Find out how experts do so by balancing diagnostic protocols against the need to respond to unique patient problems.
Overview of Disease Subtypes
Dr. Beckman has co-authored two of the five major reports offering recommendations on OSD and dry eye since 2017, including the ASCRS Cornea Clinical Committee’s 2019 consensus-based algorithm for the preoperative diagnosis and treatment of OSD1 and a “clinical guide” by the Cornea, External Disease, and Refractive Society (CEDARS) that combines the latest evidence-based approaches. Below is an overview of the five key CEDARS’ disease subtypes:
• Subtype 1: Aqueous deficiency. This primary manifestation is characterized by a reduction in lacrimal gland secretions, which form the bulk of the aqueous component of the tear film. Dr. Beckman says it may be caused by dysfunction or destruction of the lacrimal gland, or scarring and blockage of its ducts, which can prevent secretions from reaching the ocular surface. Injury, surgery, systemic conditions and topical agents can reduce corneal sensation, causing neurogenic inflammation that leads to decreased gland activity, he says.6,7 You’ll see a decreased tear lake, increased tear-film osmolarity and/or inflammation.8,9
• Subtype 2: Blepharitis/meibomian gland dysfunction (evaporative and nonevaporative). MGD can be asymptomatic or symptomatic.10 Symptomatic cases may be restricted to the lids or be associated with MGD-related OSD that includes evaporative dry eye, according to the CEDARS report.11 “Insufficient meibum flow leads to an abnormal lipid component and excessive tear evaporation,” says Dr. Beckman.6 “However, you may also see patients who have inflammation without tear evaporation.”
• Subtype 3: Goblet cell deficiency/mucin deficiency. “This subtype is based on goblet cell disease, causing mucin deficiency,” says Dr. Beckman. “These patients may have experienced a chemical burn, contact lens overwear, or have Stevens-Johnson Syndrome. Ocular medications such as glaucoma drops may cause goblet cell loss, decreasing mucins.” Conjunctival tissue can be destroyed. “Tears evaporate too quickly,” he observes. “Typically, evaporating tears make us think of lid margin disease. But not all evaporative disease is lid margin disease.”
• Subtype 4: Exposure. “Exposure affects patients who can’t completely close their eyes,” says Dr. Beckman. “They could have an incomplete blink from previous ptosis surgery, or a history of Bell’s palsy, trauma, scarring and Parkinson’s disease. You may find normal tear production and TBUT, but the tears don’t last between blinks.”12
• Subtype 5: Dysfunctional Tear Syndrome/Co-conspirators. “The term ‘co-conspirators’ refers to conditions affecting the tear film and ocular surface that may either exacerbate dry eye or masquerade as dry eye,” says Dr. Beckman. These could include superior limbic keratoconjunctivitis, medicamentosa, Thygeson’s superficial punctate keratitis, mucus fishing syndrome, contact-chemical toxicity, allergic/atopic conjunctivitis, conjunctivochalasis, ocular allergy and glaucoma drops.
Overlapping Manifestations
Although subtyping can lead to an accurate diagnosis, Dr. Beckman and others note that overlapping manifestations will complicate your investigation. The following symptoms may be found in all of the subtypes: ocular discomfort; dryness; burning; stinging; grittiness; foreign body sensation; photophobia; and blurred or fluctuating vision. Also, aqueous deficiency can overlap with blepharitis/MGD and exposure-related OSD. Blepharitis/MGD can also overlap with goblet cell deficiency, which can additionally overlap with exposure-related disease.
“Individualizing your approach to every patient is critical,” says Elizabeth Yeu, MD, a surgeon from Norfolk, Virginia who teamed with Dr. Beckman and others to write the CEDARS report. “There can be good concordance of signs and symptoms of dry eye disease or there can be misalignment.”
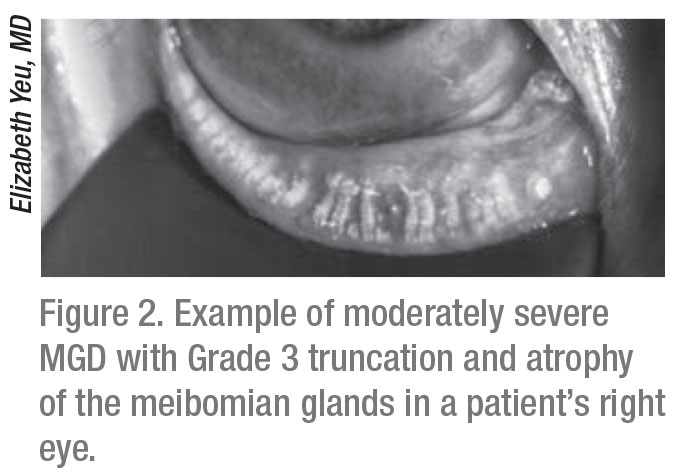 |
“Patients don’t always present with the same symptoms,” says Anat Galor, MD, associate professor of ophthalmology at the Bascom Palmer Eye Institute. “Individuals may report a variety of pain-related symptoms, such as sensations of dryness, burning or aching, or they can have visual complaints, such as poor or fluctuating vision. Different disease sub-types may underlie these symptoms.”
Getting a thorough history is critical for a patient who complains of dry eyes. Besides using three leading questionnaires—SPEED, OSDI and DEQ5, all available online—ask patients if their eyes are affected by dry-eye symptoms, including pain, eye fatigue, light sensitivity, blurred vision, poor vision and night-time driving issues.
“I have a large dry-eye population, and I find a spectrum of patient complaints and underlying findings,” says Dr. Galor. “A basic clinical exam is still the most important thing we can do.”
She checks blink rate, lid closure of both lids, laxity and lid anatomy. She evaluates TBUT, performs ocular staining and probes for comorbidities, such as arthritis,13 Sjögren’s syndrome,14 diabetes,15 ocular allergies,16 depression and anxiety.17 Also important: Ocular and systemic medications. Antihistamines, beta blockers, antispasmodics, diuretics and some psychotropic drugs reduce lacrimal secretion and may increase the potential for subtypes of dry eye.18 It’s important to remember that tear secretion rates decrease in the elderly is also important.
Dr. Yeu listens carefully to patients, mindful that some experience more than one subtype and can be affected by nerve damage, such as neuropathy of the trigeminal nerve endings associated with diabetes or other corneal manifestations.19 “They may be hyperesthetic or hypoesthetic,” she adds.
When To Test Tear Osmolarity
Most doctors evaluate tear osmolarity, a biomarker of ocular surface health. The TearLab Osmolarity System measures concentrations that range from 300 mOsm/L or below (stable) to above 340 mOsm/L (severe instability). A difference between eyes greater than 8 mOsm/L also indicates instability, even for readings below 300 mOsm/L.
Dr. Beckman uses the test to triage patients early. “Besides identifying dry eye, osmolarity helps us grade severity,” he says. Even though it doesn’t differentiate by disease subtype, osmolarity can help monitor response to treatment. “Osmolarity is volatile,” he notes. “A one-time normal reading doesn’t rule out dry eye, just as a one-time normal blood sugar reading doesn’t rule out diabetes. But consistent normal osmolarity alerts us to look for other causes of OSD symptoms.”
Dr. Galor doesn’t use osmolarity testing in clinical practice, although she values it in research, where she documents the osmolarity of study participants. In her practice, she evaluates tear stability via TBUT, and aqueous production via Schirmer’s test. She also relies on InflammaDry (Quidel) to test for the presence of metalloproteinase 9 (MMP-9), an inflammation marker found on the ocular surface.
In appropriate individuals, she uses adjuvant imaging tests such as the LipiScan (Tear Science) to evaluate meibomian gland morphology (meibography). Dr. Galor also uses in vivo confocal microscopy–both the Confoscan CS4 (Nidek) and Heidelberg Retinal Tomograph with Rostock Corneal Module (HRT-RCM). One primary use is to evaluate nerve anatomy and whether inflammatory cells are present within the cornea.
Why Advanced Testing?
Dr. Yeu says meibomian gland dropout, congestion and atrophy are essential to evaluate on infrared meibography. These findings provide insight into the presence and potential chronicity of a patient’s OSD, particularly in patients who are asymptomatic and may have otherwise “slipped through the cracks.” As a surgeon who performs 1,800 cataract procedures a year, she sees many patients for surgical evaluations. Dr. Yeu examines many of them without the benefit of prior diagnoses suggestive of OSD because referring primary care practices provide limited insights.
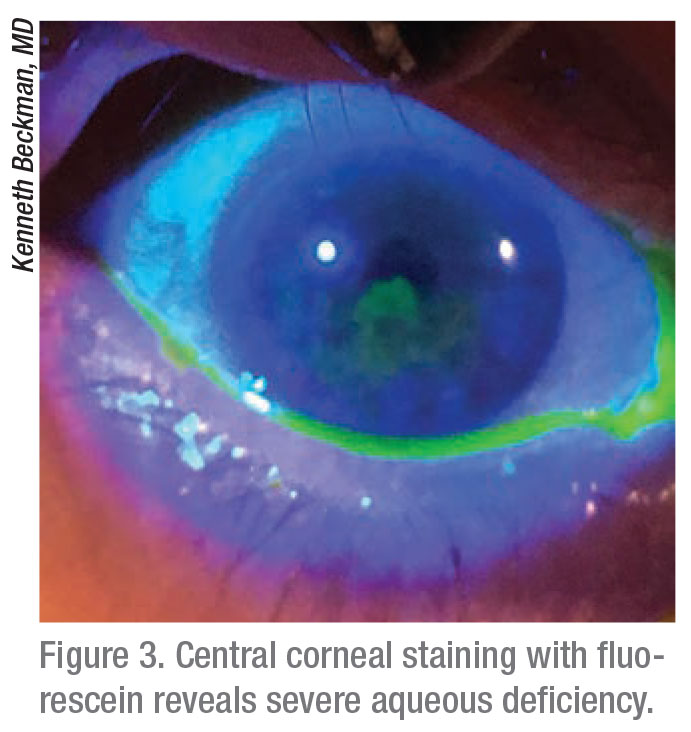 |
“I need to determine if these patients have inflammatory or tear osmolarity issues, so we objectively test patient tears,” she says. “I generally can’t test tear osmolarity and Inflamma Dry during the first visit due to reimbursement issues, so I perform a tear osmolarity test during the initial visit and an InflammaDry test during the follow-up visit.
“It’s better for me to find out if their MMP-9 is positive, especially if they’ve been treated with cyclosporine (Restasis) or lifitegrast (Xiidra). If they’re still positive for MMP-9, that tells me the treatment they’ve been receiving is not enough, as is often the case, because one medication for a disease process is often not enough.”
Positive and negative test results clarify the etiology and treatment options. Dr. Yeu trusts that a patient who has a positive InflammaDry finding will respond more favorably to an anti-inflammatory drop, including a steroid for acute cases and a steroid-sparing anti-inflammatory in chronic care.
“A negative osmolarity test result doesn’t absolutely mean that the patient is negative for OSD,” adds Dr. Yeu. “As Chris Starr, MD, and others demonstrated after completing one recent study, a negative tear osmolarity can also occur in OSD masqueraders and comorbidities, including MGD, allergic conjunctivitis and conjunctivochalasis.”20
Dr. Beckman also documents his patients’ tear osmolarity initially, then uses InflammaDry to get a quick read on his patients’ status, even if he sees no obvious signs of disease.
“If you get a positive MMP-9, you’re going to think this is an inflammatory condition,” Dr. Beckman says. “If the test is negative, reflecting lower levels of this inflammatory marker, I may not treat as aggressively with anti-inflammatories. InflammaDry helps monitor the patient. You can see over time if the MMP-9 level normalizes.”
Relying On Staining Too Much?
Dr. Yeu believes many ophthalmologists still rely too heavily on ocular surface staining as the sole way to diagnose OSD and dry eye, instead of performing a thorough ocular surface exam. “If patients sound like they have dry eye, the next step has classically been to seek out conjunctival and corneal staining,” she observes. “But if you’re going straight to the dye, then you are going to miss a whole lot of dry eye.”
As an example, she describes patients who report fluctuating vision. “This symptom often hides aqueous deficiency, which often means they have MGD because they’re not expressing enough meibum to stabilize their tear film,” she says. “They may have allergies and may be taking daily doses of Benadryl or other antihistamines, which can have an extreme drying effect even after four days of use.”
Besides taking a careful history of such a patient, Dr. Yeu checks lid margin health, including elasticity, signs of notching, capping of the glands, telangiectasia and secretion quality. “Not understanding signs and symptoms often creates a disconnect,” she adds.
She also cautions against overlooking abnormal surface architecture.
“Conjunctivochalasis, anterior membrane dystrophy, Salzmann’s nodules, pterygium and pinguecula are all mechanical elevations that break up the pre-corneal tear film,” she points out. “If a patient doesn’t have the ability to refresh the tear film, that is an issue. Alternatively, if a patient has poor sensation due to a neurotrophic component architecturally, the patient won’t report any symptoms. This can be the source of problems or exacerbate OSD.”
| Current Tests for Diagnosing and Monitoring Ocular Surface Disease |
Either alone or in combination, these tests help diagnose a patient with OSD.
|
Staining Routinely
Dr. Beckman, who also closely checks lid margins and tests the meibomian glands for normal expression, stains the eyes of nearly every OSD suspect. “I look for aqueous deficiency, suggested by staining in the interpalpebral zone, which means the eye is exposed between the lids,” Dr. Beckman says. “For evaporative disease, I typically look for rapid TBUT, a marker of that subset. I check for goblet cell/mucin deficiency. Commonly, we’ll see conjunctival scarring in later stages. For exposure, I see how quickly the patient is blinking and if it’s a complete blink. Staining typically shows around the inferior of the cornea because the lids aren’t closing all the way and the inferior cornea is getting damaged.”
Dr. Beckman says the CEDARS report showed staining patterns that are worth using as a reference. Superior staining, for example, should prompt you to look under the upper lid for signs of a foreign body or trichiasis. Other possibilities related to superior staining: floppy lid syndrome; superior limbic keratitis; blepharitis; conjunctival concretions; vernal keratoconjunctivitis; infectious keratitis; superior entropion; and atopic keratoconjunctivitis. “Superior staining also may prompt me to think of some of the co-conspirators, those conditions that can cause inflammation of the conjunctiva but don’t result from dry eye,” says Dr. Beckman.
He notes that while inferior staining often reflects exposure, “if you see inferior medial staining, leading to the punctum, the patient may have medicamentosa,” he adds.
Individualizing Diagnostics
Our experts use the latest of today’s diagnostic technologies, making choices that depend on availability and their preferences. All of them say the diagnostics they use provide the necessary rigor to reach definitive conclusions. “We now have a lot of tests,” says Dr. Beckman. “You don’t have to do everything. It’s more important to be comfortable with what you’re doing. Simple tests such as osmolarity, staining or Schirmer’s are easy to do, cheap and can make a big difference.” Dr. Yeu uses all available diagnostic tests, just not on the same day. (See “Current Tests for Diagnosing and Monitoring Ocular Surface Disease” above.) An initial exam for a new patient who isn’t a surgical referral should always include osmolarity and meibography, she says. “I’ll do the InflammaDry test. I also monitor OSD once a year with topography. When necessary, I bring the patient back six months after ordering treatment to see if the MMP-9 went down.”
Among today’s diagnostic choices are scans, imaging studies and topographers that provide insights that weren’t available to practitioners in the past. For example, meibography (LipiScan) offers images of glands that reveal just how extensive undetected disease is in young and old patients.
“You can see dilation of the meibomian gland over time, leading to atrophy and, eventually, loss of the glands,” says Dr. Beckman. “Often, when these glands are lost, they don’t regenerate. You see a thick pasty oil that’s become almost like a cement coming out of the meibomian glands. We try to salvage them. The glands need treatment so they can start producing healthier oil, or the patients’ eyes will dry out.”
Dr. Yeu agrees meibography needs to be followed over time. “Significant insult to the meibomian gland can progress for years, causing significant dropout,” she notes. “Atrophy and congestion that prevent expression of meibum contribute significantly to OSD. I’ve seen 20-something-year-olds with destruction involving more than half of their meibomian glands. But I’ve also seen healthy meibomian gland architecture in the presence of a patient’s significant meibomian gland dysfunction.
In general, destruction of the meibomian gland is a secondary process that results from poor egress of meibum. Poor egress leads to congestion of the meibomian glands, which leads to pressure atrophy and destruction of the glands. We now know that assessing the health of the entire tear film really requires us to understand meibomian gland health in particular.”
Cataract Surgery Candidates
With the recent ASCRS report on managing OSD in cataract surgery candidates,1 more surgeons appreciate that OSD can complicate surgery. So how do the experts reduce the risk?
“Patients who are contemplating cataract and refractive surgery need to complete thorough testing to help us uncover OSD,” says Dr. Yeu. She may stain the ocular surface with fluorescein to investigate the presence of abnormal morphology.Besides meibography, she also orders corneal topography and focuses on keratometry images. The views are important for evaluating the mires’ appearance, not just the K-values, she notes.
“The appearance of the rings yields good information,” she adds. “Irregular mires suggests an irregular ocular surface, either because of an issue with the tear film or the presence of conditions such as epithelial basement membrane dystrophy or subtle Salzmann’s nodular dystrophy.”
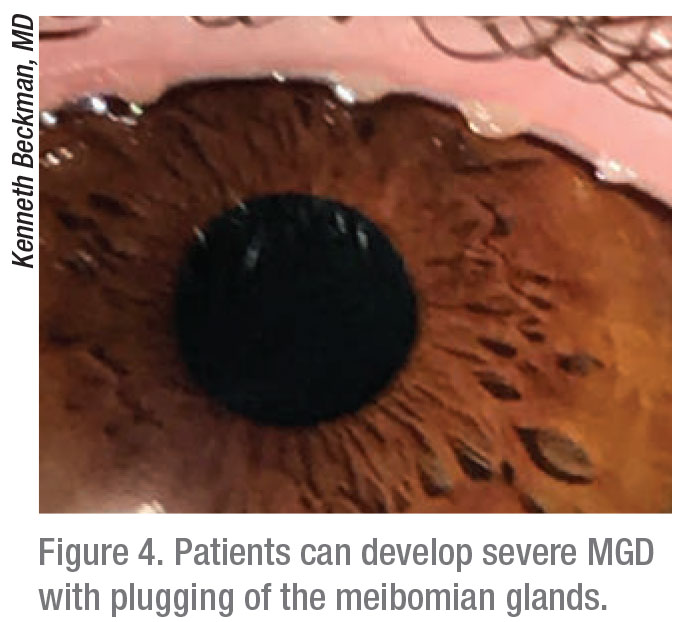 |
“For a very dry eye, you may discover mires and keratometry data—such as Atlas SimK values on an elevation map—that are dull or missing,” she observes. “In the keratometry view, instead of seeing smooth rings, you may find mires that are warped or ‘squiggly.’ An irregular tear film can also present as irregular astigmatism, and it can even be misleading enough to take on a keratoconus-like appearance.”
After diagnosing OSD, Dr. Yeu’s response depends on the goals of her patients. “If they want to proceed with surgery, I begin treatment and postpone the surgery for five to seven weeks. This allows for a follow-up of dry-eye treatment and ocular surface preparation in two to four weeks after the initial visit.”
Dr. Yeu often starts these patients on omega-3 capsules and prescribes interventional lid hygiene therapy, such as microblepharoexfoliation. “I won’t consider these patients for surgery, specifically a refractive IOL option, unless they make an agreement with me that they will manage their disease aggressively before and after surgery,” she says, noting that OSD symptoms may worsen three to six months after their procedures. “They may require a short course of acute treatments, such as steroids, or chronic steroid-sparing anti-inflammatory therapy,” says Dr. Yeu. “Identifying and
counseling patients preoperatively about any subtype of OSD is the key. Symptom questionnaires, topography readings and meibography help alert me to any issues.”
If you don’t treat preop OSD aggressively, Dr. Yeu offers these blunt words of advice: “Prepare for extreme postop IOL dissatisfaction. It’s very important to see how patients will respond to treatment before surgery.”
Meeting Refractive Challenges
Dr. Yeu says any OSD that responds poorly to aggressive treatment will limit her use of IOLs to spherical or toric monofocals. Premium IOLs split light, reduce contrast sensitivity and scatter light amid multiple points of vision. “These characteristics can disturb the vision of OSD patients,” she says. “I will also not do corneal limbal relaxing incisions. I would rather patients be extremely satisfied with one focal point with a monofocal IOL—distance, for example—instead of marginally satisfied or unhappy with multiple points of vision, especially after they’ve invested in an elective upgrade. This is a tough conversation to have, but it’s the most important part of diagnosing and managing these patients.”
Being Prepared
By incorporating this sage advice into your practice and adopting new and old tests appropriately, you can minimize the often undetected but deleterious effects of OSD on your patients. Your reward? Knowing that you’re following the latest guidelines for diagnosing OSD and still retaining your individual practice style. REVIEW
Dr. Beckman reports relevant financial relationships with the following companies: Allergan, Alcon, TearLab, Takeda, Sun, Ocular Science, Eyevance, Kala Pharmaceuticals, eyeXpress, Johnson & Johnson, Bruder, NovoLog, Bausch+Lomb and Dompé. Dr. Galor is a consultant to Allergan, NovoLog and Dompé. Dr. Yeu reports relevant financial relationships with Alcon, Allergan, Bausch+Lomb/Valeant, Bio-Tissue, J & J Vision, Kala Pharmaceuticals, Merck, Novartis, Ocular Science, Ocular Therapeutix, OcuSoft, Oyster Point Pharma, ScienceBased Health, Shire, Sight Sciences, Sun Pharma, Surface Pharmaceuticals, Topcon, TearLab, TearScience and Zeiss.
1. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg 2019;45:5:669-684.
2. Epitropoulos AT, Matossian C, Berdy GJ, et al. Effect of tear osmolarity on repeatability of keratometry for cataract surgery planning. J Cataract Refract Surg 2015;41:8:1672-1677.
3. Rynerson JM, Perry HD. DEBS - a unification theory for dry eye and blepharitis. Clin Ophthalmol 2016;2455-2467.
4. Dry Eye Syndrome Preferred Practice Pattern, American Academy of Ophthalmology, 2018.
5. Craig JP1, Nelson JD2, Azar DT3, et al, TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15:4:802-812
6. Fiscella RG. Understanding dry eye disease: A managed care perspective. Am J Manag Care 2011;(17):S432-S439.
7. Stern ME, Schaumberg CS, Pflugfelder SC. Dry eye as a mucosal auto-immune disease. Int Rev Immunol 2013; 32:19-41.
8. Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol 1966;5:264-276.
9. Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci 2004; 45:4302-4311.
10. Donnenfeld ED, Solomon R, Roberts CW, et al. Cyclosporine 0.05% to improve visual outcomes after multifocal intraocular lens implantation. J Cataract Refract Surg 2010;36:1095-1100.
11. Milner MS, Beckman KA, Luchs J. Dysfunctional tear syndrome: Dry eye and associated tear film disorders–new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;Suppl 1:3-47.
12. Tsubota K, Nakamori K. Effects of ocular surface area and blink rate on tear dynamics. Arch Ophthalmol 1995; 113:155-158.
13. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:2:75-92.
14. Begley CG, Chalmers RL, Abetz L. The relationship between habitual patient reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci 2003;4411:4753-61
15. Yoo TK, Oh E2.Diabetes mellitus is associated with dry eye syndrome: A meta-analysis. Int Ophthalmol 2019; May 7. [Epub ahead of print].
16. Bielory L. Allergic conjunctivitis: the evolution of therapeutic options. Allergy Asthma Proc 2012;33:2:129-39.
17 Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye (Lond). 2016;30(:2:1558-1567
18. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf 2017;15:334-65.
19. Liu Y, Chou Y, Dong X, et al. Corneal Subbasal nerve analysis using In vivo confocal microscopy in patients with dry eye: Analysis and clinical correlations. Cornea 2019; Jul 10. [Epub ahead of print]
20. Brissette AR, Drinkwater OJ, Bohm KJ, Starr CE. The utility of a normal tear osmolarity test in patients presenting with dry eye disease like symptoms: A prospective analysis. Cont Lens Anterior Eye. 2019;42:2:185-189.



