Tear dysfunction syndromes such as Sjögren’s, evaporative dry eye or aqueous-deficient dry eye represent a spectrum of ocular disorders with a huge impact on vision, ocular health and quality of life. Efforts to develop new therapies to address these conditions face a daunting gauntlet of intrinsic and extrinsic factors that modulate tear production, tear composition and tear function.
While there are a number of tools available for evaluating tears, many seem to fall short in terms of their ability to report reliably and reproducibly on the changing attributes of tear physiology that underlie dry-eye diseases. This month we examine the many ways in which tears are measured, and consider the degrees to which these many metrics measure up.
Tears 101
The tear film is an amalgam of ingredients derived from three different sources: goblet cells; meibomian glands; and lacrimal glands.1 The conjunctival goblet cells provide the primary source for the glycoprotein conjugates called mucins that lubricate the ocular surface. Lipids and wax esters from meibomian glands provide a hydrophobic sealant to retard aqueous evaporation. The largest component of the tears comes from the primary and accessory lacrimal glands, which secrete an aqueous mixture of salts, protein and water. The combination of ingredients from these three sources acts as a physical buffer, cleaning fluid and source of nutrients for the underlying cornea and conjunctiva.
Lacrimal secretions are mixtures derived from two cell types found within the acini of the glands. Serous cells form acini that secrete electrolytes and mixtures of many different proteins (estimates suggest 200 to 300 different polypeptides in humans). The release of salts such as Na+, K+, Cl- and Ca2+ provides the osmotic force that pulls water from the gland, forming the bulk of the tear volume.1 Smaller numbers of mucus-secreting acini are also present, secreting soluble mucins such as Muc7. Additional tear components, notably the IgA and IgG antibodies, are secreted by plasma and epithelial cells within the lacrimal gland. The specific protein content of lacrimal secretions varies significantly depending on the nature of the stimulatory input.
Both basal and reflex tearing occur in response to autonomic inputs, including stimulation of parasympathetic (via transmitters acetylcholine and VIP) and sympathetic (norepinephrine) nerves.2 In addition there is evidence that ATP and/or adenosine may act as a positive modulator of lacrimal secretion via P2Y receptors, perhaps via an effect on electrolyte secretion.3 Higher-order lacrimal secretion control comes from several sources, including central regulation of basal activity and reflexive responses to environmental stimuli via ocular sensory inputs. These are processed by way of the trigeminal nucleus to parasympathetic and sympathetic tracts which feed back to the lacrimal glands, as well as conjunctival sites (including goblet cells) and meibomian glands. Studies in mouse models provide compelling evidence that it is the temperature-sensitive corneal sensory nerves that regulate basal lacrimation, providing a set point of secretory stimulation that is exquisitely sensitive to small changes in corneal surface temperature.4
| Table 1. Comparison of Methods of Tear Measurement |
|
| Tear production metrics |
Tear volume |
Tear turnover |
Reliability
|
Reproducibility
|
Correlation with signs/symptoms |
| Schirmer's 1 |
good |
fair |
good |
fair |
fair |
| Schirmer's II (with anesthetic) |
fair |
good |
good |
good |
fair |
| phenol red thread test |
good |
fair |
good |
fair |
fair |
| meniscus height |
best |
good |
good |
best |
good |
| fluorophotometry |
best |
best |
best |
good |
best |
|
|
A similar sensory circuit provides input from the upper and lower eyelids, which then feeds back to the orbicularis oculi and levator palpebrae muscles that control blinking.
2 It’s worth remembering that a blink exerts several effects on the aqueous tear film: redistribution; drainage; and the pressure that causes meibomian gland secretion. A number of studies have established that a primary means of compensation for patients with reduced aqueous tear production is altered blink rate.
5,6
Regulating Tear Flow
Layered upon the basal level of tear secretion is a stimulated component that is a response to external and internal factors including diurnal patterns, environmental fluctuations and physiological status. Diurnal changes in tear composition, particularly the variation in the variety and concentration of tear proteins, are well-established.7 Early studies suggested that the nocturnal tear film lacks a significant reflex tear component, so tear protein levels increase over the course of the time period when the eyes are closed.8 Additional diurnal changes may underlie daily variation in visual acuity, particularly in those who suffer from dry eye. Most patients with dry-eye disease typically report that their symptoms worsen as the day progresses.9
Environmental effects provide the most significant impact on a patient’s reflex tearing.10 Tear flow is stimulated beyond basal levels in response to wind, heat or decreases in relative humidity. The inability to respond appropriately to stimuli such as wind or dryness describes a large segment of the dry-eye population.
Other environmental factors such as light-induced alterations in blink behavior can also have substantial impact on tear turnover and tear-film stability. In addition, there are tear reflex stimuli triggered by either nasal or oral sensory stimuli (one has but to consider the effects of the humble onion). Overall, reflex or stimulated tearing comprises well over half of the total tear volume, and is a key to homeostatic maintenance of tear-film stability and ocular health.
| Non-invasive measurement is key: Because of the sensitivity of feedback inputs, the issue of reflex tearing is a major hurdle to any successful assessment of aqueous output. |
|
In addition to external factors, systemic physiological factors can also impact the flow of aqueous tears. There is evidence that in older subjects, reduction in whole body hydration can lead to reduced aqueous flow and a concentration of tear fluid components.
11 Interestingly, at least one study suggests that younger individuals have the ability to compensate and produce normal tear volumes, even in cases of dehydration.
12 In addition, there are many over-the-counter and prescription drugs (and perhaps herbal, holistic therapies) that carry with them the baggage of “anti-cholinergic” side effects and the decreases in all types of secretory activity which that entails.
13 A classic example is described in a study we did in 2007 that showed some systemic antihistamines can exacerbate the signs and symptoms of dry eye by causing a reduction in aqueous tear production.
14
Two other key physiological factors that can impact tear flow are the production and secretion of meibum and mucin to complete the triad of components that comprise the tear film. A lack of sufficient meibum, in particular, can alter evaporative properties and result in a reduction of aqueous tears. This highlights the conundrum that while we strive to isolate the specific causes of our patients’ dry eye—aqueous deficiency, evaporative dry eye, Sjögren’s syndrome or MG disease—the interdependence of each facet of the tear film limits our ability to focus treatment on a single underlying defect.
Measuring Aqueous Output
A host of techniques are available for assessing aqueous tear production, for quantifying tear-film properties and for measuring rates of tear turnover.15 Each method has advantages and disadvantages but the key hurdle in studies of aqueous tear dysfunction is the disconnect between objective measures and symptomatic disease.
There is a growing realization that while simple tests such as Schirmer’s or the phenol red thread may provide a measure of tear output suitable for a clinical evaluation, they don’t provide a sufficient level of sensitivity or reproducibility to be applied to drug discovery efforts. Evaluation techniques such as the measurement of tear meniscus height or fluorophotometry appear to be better suited for studies in which a specific metric of tear production is needed.
Meniscus measures can be done with a slit lamp, although they are now more often measured using OCT. Both approaches benefit from being relatively non-invasive. This non-invasiveness is key: Because of the sensitivity of feedback inputs, the issue of reflex tearing is a major hurdle to any successful assessment of aqueous output.15,16 Meniscus height can be a useful tool to follow changes in an individual, but any population assessment must normalize data to measure relative change. In addition, surface tension issues can significantly change the values obtained by standardized methods and these are subject to fluctuation depending upon the concentration of meibum and mucin, and even on the osmolarity of the tear film.
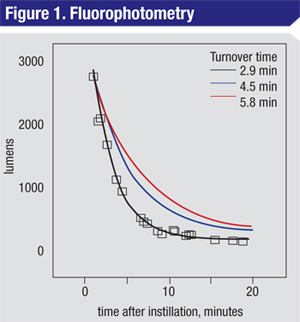 |
|
A plot of fluorophotometry data for a normal patient (black squares, line) shows the relationship between fluorescence signal (lumens) as a function of time. The derived tear-turnover rate from this curve fit is 2.9 minutes. The two hypothetical curves shown are for individuals with a decreased turnover rate 1.5 times (blue) or two times (red) slower than the rate derived from this normal patient. These decay curves would be expected for patients with reduced tear turnover. |
|
An evaluation of tear output metrics in the DEWS report states, “For studying the tear film, the greatest opportunity lies in the use of noninvasive techniques involving the sampling of optical radiation reflected from the tear film.”
16 One such non-invasive approach is fluorophotometry (FP), a technique that measures the rate at which tears on the ocular surface are replaced.
17
Fluorophotometry, which is sometimes referred to as tear turnover, uses a fluorescent tracer in the tears and follows the decline of tracer concentration in tears over time. By measuring the kinetics of this process it’s possible to derive values for tear turnover, total tear volume and tear “flow rate.” While the equipment needed makes the process prohibitively expensive for use in a general practitioner’s office, the reliability and non-invasive nature of the measure suggest that it should be the metric of choice for precise assessment of aqueous production in clinical research.
Homing in on Flow Rates
Like many clinical tools used by the dry-eye diagnostician, FP can be an outstanding evaluation device once the critical parameters are identified and optimized.
In terms of the mechanics of performing FP, a small volume (≈1 µl) of tracer fluorophore is applied to the conjunctival fornix. After a brief delay, the ocular surface is scanned for a luminescence signal at regular intervals for 20 to 30 minutes. The decay in the signal represents the continuous dilution of the fluorophore in the tear volume; by measuring the rate of that decay it’s possible to generate a value for the tear turnover rate, typically in the range of two to four minutes (See Figure 1, at left). Extrapolation to a theoretical zero point can also yield a value for the patient’s total tear volume, but the real value in FP may be in its ability to follow changes in turnover rates before and after test compounds.
Studies conducted at our research firm, Ora Inc., have refined the protocols used for FP in order to improve reproducibility while reducing the variability of the method. These improvements include ergonomic optimization during measurements, as well as adjustments to the volume and concentration of fluorophore that’s used for the measurement. With these refinements, FP can be an invaluable tool in clinical studies of dry-eye therapies, either as an inclusion criterion, a clinical endpoint following the clinician’s therapeutic intervention, or both.
A comparison of dry-eye metrics (See Table 1, p. 42) suggests that FP has high sensitivity and specificity, and is superior to the other well-known measures of tear production in terms of its predictive value. Simply stated, FP displays a superior ability to correlate with other signs and symptoms of dry eye such as corneal fluorescein staining and ocular surface disease index survey data. The biggest challenge to the use of FP as a metric going forward is the need for more studies; it’s possible that FP may be of less predictive value with some forms of dry-eye disease, but considering the complexity of aqueous tear-film regulation this challenge is best met by an empirical approach.
It’s likely that a combination of the current standards of ocular surface staining and ocular surface disease index surveys, in combination with objective metrics such as FP, will provide the jump start needed to gain traction in the search for new dry-eye therapies. REVIEW
Dr. Abelson is a clinical professor of ophthalmology at Harvard Medical School and senior clinical scientist at the Schepens Eye Research Institute. Ms. Kelley and Dr. McLaughlin are medical writers at Ora Inc.
1. Beuerman RW, Mircheff A, Pflugfelder SC, Stern ME. The lacrimal functional unit. In: Pflugfelder SC, Stern ME, and Beuerman RW, eds. Dry Eye and Ocular Surface Disorders. New York: Marcel Dekker, 2004:11-39.
2. Duke-Elder S, Wybar KC. The Anatomy of the Visual System. London: Henry Kimpton, 1961:768.
3. Kamada Y, Saino T, Oikawa M, Kurosaka D, Satoh Y. P2Y purinoceptors induce changes in intra-cellular calcium in acinar cells of rat lacrimal glands. Histochem Cell Biol 2012;137:1:97-106.
4. Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature Medicine 2010;16:1396-1399.
5. Abelson R, Lane KJ, Rodriguez J, Johnston P, Angjeli E, Ousler G, Montgomery D. A single-center study evaluating the effect of the controlled adverse environment (CAE) on tear-film stability. Clin Ophthalmol 2012;6:1865-1872.
6. Ousler GW, Abelson MB, Nally LA, Welch D, Casavant JS. Evaluation of the time to “natural compensation” in normal and dry eye subject populations during exposure to a controlled adverse environment. In: Sullivan, DA, Stern ME, Tsubota K, et al, eds. Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3. New York: Kluwer Academic/Plenum P, 2002:1057-1063.
7. Walker PM, Lane KJ, Ousler GW, Abelson MB. Diurnal variation of visual function and the signs and symptoms of dry eye. Cornea 2010;29:6:607-12.
8. Sack RA, Tan KO, Tan A. Diurnal tear cycle: Evidence for a nocturnal inflammatory constitutive tear fluid. Invest Ophthalmol Vis Sci 1992;33:3:626-40.
9. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf 2007;5:2:75-106.
10. Tsubota K. Tear dynamics and dry eye. Prog Retin Eye Res 1998;17:4:565-96.
11. Walsh NP, Fortes MB, Raymond-Barker P, Bishop C, Owen J, Tye E, Esmaeelpour M, Purslow C, Elghenzai S. Is whole-body hydration an important consideration in dry eye? Invest Ophthalmol Vis Sci 2012;53:6622-7.
12. Walsh NP, Fortes MB, Esmaeelpour M. Influence of modest changes in whole-body hydration on tear fluid osmolarity: Important considerations for dry eye disease detection. Cornea 2011;30:1517; author reply 1517-8.
13. Pappano AJ. Anti-cholinergic Drugs. In Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology, 11th edition. New York: McGraw Hill, 2009:113-126.
14. Ousler GW, Workman DA, Torkildsen GL. An open-label, investigator-masked, crossover study of the ocular drying effects of two antihistamines, topical epinastine and systemic loratadine, in adult volunteers with seasonal allergic conjunctivitis. Clinical Therapeutics 2007;29:611-616.
15. Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the study of ocular surface disease. Ocul Surf 2005;3:3:143.
16. Methodologies to diagnose and monitor dry eye disease: Report of the diagnostic methodology subcommittee of the international dry eye workshop. Ocul Surf 2007;5:2:108-152.
17. Fahim MM, Haji S, Koonapareddy CV, Fan VC, Asbell PA. Fluorophotometry as a diagnostic tool for the evaluation of dry eye disease. BMC Ophthalmol 2006;6:20.