In this article, I’ll review the indications for the use of a keratoprosthesis, list the available devices and outline the results you can expect to achieve with them.
KPro Indications
Diseases affecting the cornea are a major cause of global vision loss, second only to cataract in overall importance, and both inflammatory and infectious etiologies resulting in corneal scarring can cause functional blindness.3 Globally, bilateral corneal blindness is estimated to be 4.9 million persons, or 12 percent of the total 39 million blind, based on the World Health Organization’s 2010 global blindness data.4 A KPro, however, can reverse corneal blindness and restore eyesight, and it’s most useful in cases where conventional surgical techniques such as penetrating keratoplasty or limbal stem cell transplantation are most often projected to fail due to the adverse ocular surface conditions that prevail in a subset of patients with corneal blindness. Recent data appear to
 |
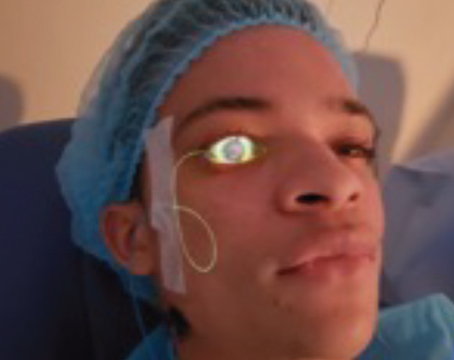
| Figure 1. Slit-lamp photograph of a Boston Kpro in a highly vascularized cornea with multiple prior failed corneal grafts. |
The clinical indications for a keratoprosthetic device such as the BKpro include refractory corneal blindness with very poor prognosis for conventional penetrating keratoplasty, such as failed corneal grafts, autoimmune ocular disorders such as Stevens-Johnson syndrome, toxic epidermal necrolysis, herpetic keratitis, congenital anomalies such as aniridia; chemical or thermal injury and pediatric corneal opacities.
BKPro Type II is especially indicated in the rarer cases of symblepharon, severe dry eyes and significant corneal scarring associated with autoimmune and inflammatory diseases. Recent reports indicate type II BKPro is indicated most commonly for Stevens-Johnson syndrome (41.7 percent) and mucous membrane pemphigoid (41.7 percent).5
Patients who have a high risk of failure with conventional penetrating keratoplasty, and therefore might require an artificial cornea, fall into two categories: those with optimal tear function and those who don’t have lubricating tears and therefore run the risk of surface keratinization and corneal blindness.
The BKPro
Globally, the BKPro has the highest ocular implantation rate,6 with more than 12,000 devices implanted worldwide as of March 2015.7 This device was initially developed at the Massachusetts Eye and Ear Infirmary in 1960s, and was Food and Drug Administration-approved in 1992 and CE-marked in 2014.
Other types of keratoprostheses of interest include: AlphaCor artificial cornea; osteo-odonto keratoprosthesis (OOKP);8 KeraKlear (Keramed); Micro Cornea;9 Auro KPro (BKPro type 1-based KPro);10 LVP KPro (Auro KPro with a longer optical stem);11 and the MICOF (from Russia’s Moscow Eye Micro Surgery Complex).12 The AlphaCor was FDA approved in 2002, while the rest aren’t approved in the United States.
The BKPro consists of model types I and II, the former being used in patients with adequate tear function and the latter being used in ocular conditions with no surface tears and the presence of a keratinized ocular surface, where the device is placed through the eyelids to provide vision in an otherwise functionally blind eye.
The device has a collar-button configuration; the two main parts of the BKPro are the front plate with its integral optical cylinder or optical stem (3.35-mm diameter) that’s made of poly(methyl methacrylate), and the backplate that can be either PMMA or titanium. The recent introduction of an enlarged titanium backplate to clamp the donor-host junction is aimed at decreasing the rate of RPM. However, currently there is no definitive comparative evidence that a titanium backplate is superior to a PMMA backplate in reducing RPM formation. Because of this, the PMMA backplate is still a viable surgical option due to its relatively long-term track record of proven safety.
While the PMMA backplate is cosmesis-neutral for the most part, the blue titanium
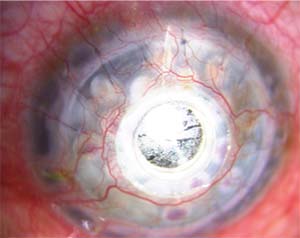 |
| Figure 2. A Boston Kpro with a thick retro-prosthetic membrane. |
A titanium backplate is thinner than a PMMA model, and therefore occupies less anterior chamber space, is considered to be more tissue-friendly, and is thought to be associated with less RPM than the medical-grade, transparent, biologically inert PMMA, which has been associated with many postoperative complications.13 In contrast, titanium has been shown to have superior biocompatibility and demonstrates increased corrosion resistance compared to other materials.14,15
The assembly of a BKPro consists of a donor cornea sandwiched between the front plate and backplate of the device. The central rigid optical cylinder of the BKPro passes through the central, surgically created 3-mm opening in the donor cornea. The front plate diameter is 5.5 to 7 mm,16 while the backplate is available in adult and pediatric sizes—8.5- and 7-mm diameters, respectively.17 The backplate of the adult Boston KPro contains 16 1.3-mm diameter holes, while the pediatric model has eight. These openings provide a pathway for nutrition and hydration for the donor corneal graft.
There are two types of backplate attachments: the newer click-on type that clicks onto the optic of the front plate and is made of titanium; and the older snap-on type of backplate that snaps onto the optic and is further secured with a locking ring. The newer model is threadless, while the older model is threaded on. The former’s backplate is 8.5 mm in diameter, while the older model comes in either a 7- or 8.5-mm diameter backplate made of either PMMA or titanium.
Once the Boston KPro and the donor cornea are
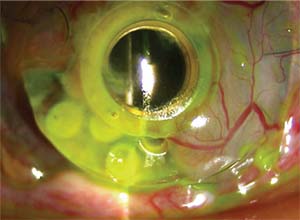 |
| Figure 3. Slit-lamp photograph displaying corneal melt with significant PMMA backplate exposure and partial forward tilt of a Boston Kpro. |
Surgical Pearls
Centration of the BKPro is important both for the donor and recipient. Recipient corneal centration of the trephination may be achieved by measuring with surgical calipers in a way similar to that used when performing a PK. For the donor cornea, the full-thickness trephination is carried out in the usual surgical manner for donor corneal disc preparation. It’s important that the central 3-mm trephination be performed in the center of the donor corneal disc. This ensures that the Boston KPro achieves uniform centration with the donor corneal disc. A device I helped design, the John Centration Ring (ASICO), is helpful in centering the central 3-mm trephination on the donor corneal disc.
To help with visualization, you can dye the donor cornea blue by immersing it in trypan blue, followed by BSS irrigation. This facilitates visualization of the donor disk during the central trephination and while suturing the assembled KPro donor corneal disk unit to the recipient peripheral corneal rim.
Suture the donor corneal rim and the recipient cornea using interrupted 10-0 nylon sutures, making sure that the surface at the donor-recipient junction is uniform without any step formation, to ensure postoperative uniform tear-film distribution on the newly created ocular surface.
Hemostasis is essential before the Boston KPro is sutured onto the recipient corneal rim. Using fine-needle-tip cautery can help achieve effective hemostasis of the recipient corneal rim, as most of these corneas may be associated with extensive corneal neovascularization. Prevent any blood from entering the vitreous cavity, as postoperative clearance of the introduced hemorrhage can be slow and often has a direct detrimental effect on the patient’s immediate postoperative vision and visual recovery.
The use of a postoperative large contact lens, such as the Kontur lens (Kontur Kontact Lens Co.), is important to keep the Boston KPro surface clean and smooth, which results in the best post-surgical visual acuity.
Results and Complications
The spectrum of postoperative complications associated with artificial corneas include RPM (Figure 2), corneal melt (Figure 3), corneal infection, extrusion of the KPro, secondary glaucoma and endophthalmitis.
A multicenter Boston KPro study published in 2006 featured a large series with 141 Boston type I keratoprostheses comprising 39 different surgeons from 17 surgical sites. The researchers reported an overall Boston KPro retention rate of 95 percent, and more than half of the patients (57 percent) had a best corrected visual acuity of equal to or better than 20/200. Postoperative complications included elevated intraocular pressure in 15 percent of patients, retroprosthetic membranes in 25 percent and sterile vitritis in 5 percent.19 None of the cases had endophthalmitis following Boston KPro implantation.
In another study, the incidence of endophthalmitis associated with the Boston KPro was 3.67 percent (five of 136 eyes) and the average time to development of endophthalmitis was 5.62 months (range: two days to eight months).20
Modified KPro
Recently, researchers at the LV Prasad Eye Institute in India attempted to use a modified type I Boston KPro, called the LVP KPro, in severe dry eye that normally would be an indication for Boston type II KPro or the OOKP.
In the trial, surgeons first covered the ocular surface of these eyes with a mucous membrane graft21 to provide a stable epithelial cover for the device. In order to accommodate the extra thickness of the mucous membrane graft, the researchers lengthened the optical stem.21 This increased stem length provides extra space around the stem beneath the front plate where the edges of the mucous membrane graft remain tucked under the front plate of the optical cylinder.21 The study’s authors reported survival of the device for more than a year without any surface breakdown, infection, extrusion or glaucoma. Larger-scale studies with longer follow-up times should provide a better idea of the safety and efficacy of the LVP KPro.
Boston keratoprosthesis has contributed significantly to the overall retention and successful visual restoration in cases of corneal blindness that are at high risk for failure of a conventional PK. Continued innovation and modifications in the surgical technique will improve the procedure’s outcomes, increase device retention times and, hopefully, decrease complications such as secondary glaucoma, RPM, infection, corneal melts and device extrusion. REVIEW
Dr. John is a clinical associate professor at Loyola University at Chicago, and is in private practice in Oak Brook, Tinley Park, and Oak Lawn, Illinois. He can be reached at 708-429-2223; fax: 708-429-2226; e-mail: tjcornea@gmail.com. He receives a small royalty on his devices from ASICO.
1. Chirila TV, Hicks CR. The origins of the artificial cornea: Pellier de Quengsy and his contribution to the modern concept of keratoprosthesis. Gesnerus 1999;56:1-2:96-106.
2. Pellier de Quengsy, G. Précis au cours d’operations sur la chirurgie des yeux. Paris: Didot, 1789.
3. Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Org 2001;79:214-21.
4. Oliva MS, Schottman T, Gulati M. Turning the tide of corneal blindness. Indian J Ophthalmol 2012;60:423–427.
5. Lee R, Khoueir Z, Tsikata E, Chodosh J, Dohlman CH, Chen TC: Long-term visual outcomes and complications of Boston Keratoprosthesis Type II implantation. Ophthalmology 2017;124:27-35.
6. Aldave AJ, Sangwan VS, Basu S, et al. International results with the Boston type I keratoprosthesis. Ophthalmology 2012;119:1530–8.
7. Salvador-Culla B, Kolovou PE: Keratoprosthesis: A Review of Recent Advances in the Field. J Funct Biomater 2016;7:2.
8. Strampelli, B. Osteo-odontocheratoprotesi. Ann Ottalmol Clin Ocul 1963;89:1039–1044.
9. Schrage NK, Hille K, Cursiefen C. Current treatment options with artificial corneas: Boston Kpro, Osteo-odontokeratoprosthesis, Micro Cornea and KeraKlear. Ophthalmologe 2014;111:1010–1018.
10. Venugopal A, Rathi H, Rengappa R, Ravindran M, Raman R. Outcomes after auro keratoprosthesis implantation: A low-cost design based on the Boston Keratoprosthesis. Cornea 2016;35:1285-1288.
11. Basu S, Sureka S, Shukla R, Sangwan V: Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Rep 2014 Mar 24.
12. Huang, Y, Yu J, Liu L., Du G, Song J, Guo H. Moscow eye microsurgery complex in Russia keratoprosthesis in Beijing. Ophthalmology 2011;118:41–46.
13. Traish AS, Chodosh J. Expanding application of the Boston type I keratoprosthesis due to advances in design and improved postoperative therapeutic strategies. Semin Ophthalmol 2010; 25:239–243.
14. Long M, Rack HJ. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998;19:1621–1639.
15. Morra M, Cassinelli C. Biomaterials surface characterization and modification. Int J Artif Organs. 2006;29:824–833.
16. Ilhan-Sarac O, Akpek EK. Current concepts and techniques in keratoprosthesis. Curr Opin Ophthalmol 2005;16:246–250.
17. Dohlman C, Harissi-Dagher M, Graney J. The Boston keratoprosthesis: A new threadless design. Digit J Opthalmol 2007;13:1–6
18. Sayegh RR, Avena Diaz L, Vargas-Martin F, Webb RH, Dohlman CH, Peli E. Optical functional properties of the Boston keratoprosthesis. Invest Ophthalmol Vis Sci. 2010;51:857–863.
19. Zerbe BL, Belin MW, Ciolino JB. Results from the multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology 2006; 113:10:1779.
20. Chhablani J, Panchal B, Das T, Pathegay A, Motukupally SR, Pappuru RR, Basu S, Sangwan V. Endophthalmitis in Boston keratoprosthesis: Case series and review of literature. Int Ophthalmol 2015;35:673-678.
21. Basu S, Sureka S, Shukla R, Sangwan V. Boston type 1 based keratoprosthesis (Auro Kpro) and its modification (LVP Kpro) in chronic Stevens Johnson syndrome. BMJ Case Reports 2014; bcr2013202756. Published online 2014 Mar 24. Accessed 6 June 2017.