A new study from researchers in
"This study is important because it is the first to show that an artificially fabricated cornea can integrate with the human eye and stimulate regeneration," said senior author May Griffith, PhD, of the Ottawa Hospital Research Institute, the
More than a decade ago, Dr. Griffith and her colleagues began developing biosynthetic corneas in
Together, they initiated a clinical trial in 10 Swedish patients with advanced keratoconus or central corneal scarring. Each patient underwent surgery on one eye to remove damaged corneal tissue and replace it with the biosynthetic cornea, made from synthetically cross-linked recombinant human collagen. Over two years of follow-up, the researchers observed that cells and nerves from the patients' own corneas had grown into the implant, resulting in a "regenerated" cornea that resembled normal, healthy tissue. Patients did not experience any rejection reaction or require long-term immune suppression, which are serious side effects associated with the use of human donor tissue. The biosynthetic corneas also became sensitive to touch and began producing normal tears to keep the eye oxygenated. Vision improved in six of the 10 patients, and after contact lens fitting, vision was comparable to conventional corneal transplantation with human donor tissue.
"We are very encouraged by these results and by the great potential of biosynthetic corneas," said Dr. Fagerholm. "Further biomaterial enhancements and modifications to the surgical technique are ongoing, and new studies are being planned that will extend the use of the biosynthetic cornea to a wider range of sight-threatening conditions requiring transplantation."
Silicone May Ease Complications of Cancer Treatment
Scott Oliver, MD, an assistant professor at the University of Colorado School of Medicine, is testing a treatment for eye cancer that may reduce vision loss associated with radiation treatment for the disease. Dr. Oliver has discovered that silicone oil applied inside the eye can block up to 55 percent of harmful radiation, enough to prevent blindness in most patients.
His findings, published in the July issue of the Archives of Ophthalmology, may revolutionize the way eye cancer is treated.
"If you get diagnosed with eye cancer you want to know, 'Is this going to kill me? Is this going to make me go blind?' " said Dr. Oliver, director of the
Dr. Oliver focused on choroidal melanoma of the eye or uveal cancer, the most common and dangerous form of a disease that strikes more than 2,000 people each year. It can spread quickly to the liver and lungs, which is often fatal. The cancer can occur in people of any age; fair skin and sun exposure are thought to be a leading cause.
Treatment often involves plaque brachytherapy in which a gold cap containing radioactive seeds is attached to the sclera. For one week the radiation slowly incinerates the tumor, but it also causes long-term damage.
"Radiation injures blood vessels and nerves in the back of the eye," Dr. Oliver said. "Half of all patients are legally blind in three years in the treated eye."
In his quest to save their eyesight, Dr. Oliver experimented with a series of substances that would block radiation from striking critical structures while allowing it to hit the tumor. He discovered that silicone oil could screen out a majority of harmful radiation.
"You don't have to block out all the radiation to protect the eye because the vital structures in the eye can tolerate low doses of radiation," he said.
Dr. Oliver experimented on cadaver eyes and tested the oil on animals in the laboratory and found no harmful side-effects.
"We are now at the point where we can embark on a clinical trial," he said. "This is a significant development in the way we treat this disease. In the past, we could save the eye with radiation but we saved vision only half the time. With this treatment, I believe we will do much better in the future."
Discovery Could Yield Myopia Treatments
Scientists at
Often the discovery of a gene still means that many years could pass before a treatment becomes available. However, gene therapies are already working well in some eye conditions, and myopia may be a good candidate condition for gene repair.
"The eye is already an organ of choice for gene therapy, for example, because the eye's small volume and self-contained area allow the therapy to remain inside the eye in a concentrated volume," said lead author Terri Young, MD, professor of ophthalmology, pediatrics and medicine, and a researcher in the Center for Human Genetics at Duke. "In addition, the eye's accessibility lets clinicians observe the effects of treatment over time with noninvasive methods that can illuminate and test the retina and other eye structures."
There is an antidote for the condition. "People need to go outside and look to the horizon," Dr. Young said. "Today's near work forces our eyes to constantly be in tension to focus on near objects – reading papers and watching monitors. We also watch TV, work in cities with high buildings, drive in heavy traffic, and generally have fewer chances for distant views, especially in urban areas. These factors affect children with developing vision, as well as many adults."
Working with a large group of researchers, Dr. Young and colleagues found several distinct spellings of DNA code near the RASGRF1 gene that had a strong association with focusing errors in vision. These findings were validated in six other Caucasian adult groups in a total of 13,414 subjects.
"Because RASGRF1 is highly expressed in neurons and the retina, it is crucial to retinal function and visual memory consolidation," Dr. Young said.
When the scientists created mice that were missing the correct gene, these mice showed changes in their eye lenses. "This was biologically convincing," Dr. Young said. "The RASGRF1 provides a novel molecular mechanism to study so that we can work to prevent the most common cause of visual impairment."
Ista Begins Phase III Program for Dry Eye
Ista Pharmaceuticals has initiated a Phase III clinical program of the company's proprietary formulation of Remura (bromfenac ophthalmic so-lution for dry eye) for alleviating the signs and symptoms of dry-eye disease. The studies are being conducted under a Special Protocol Assessment agreed upon with the FDA.
Ista plans to assess the safety and efficacy of Remura in alleviating the signs and symptoms of dry-eye disease by conducting four randomized, double-masked, placebo-controlled Phase III studies. The recently initiated Phase III studies will evaluate the efficacy and safety of bromfenac in two simultaneous studies operating under a common protocol in approximately 1,000 patients with mild or moderate dry-eye disease. The multicenter trials will be conducted at more than 30 sites in the
The two remaining Phase III safety studies are the subject of additional SPAs currently under review by the FDA. To meet FDA guidance on drugs for chronic dosing, the company expects to conduct both a six-month and a 12-month safety study. Ista anticipates it will initiate one or both of these safety studies later this year, subject to reaching agreement with the FDA on the SPAs.
Angiogenesis Promoter Identified
A new study describes how a carbohydrate-binding protein, galectin-3, promotes angiogenesis. Targeting the protein, scientists identified two approaches that significantly reduced angiogenesis in mice. These discoveries, published online August 16 in the Journal of Experimental Medicine, may lead to novel treatments for diseases caused by excessive angiogenesis, including age-related macular degeneration, cancer and diabetes.
A growing body of research indicates that a protein called galectin-3 promotes angiogenesis, indicating that it may be a valuable target for drugs that halt harmful blood vessel growth. Until now, though, scientists did not understand how galectin-3 promotes angiogenesis.
Led by Noorjahan Panjwani, PhD, researchers propose a mechanism that explains how galectin-3 brings about angiogenesis. Dr. Panjwani is a professor in the department of ophthalmology at Tufts University School of Medicine and a member of the biochemistry and cell, molecular and developmental biology program faculties at the Sackler School of Graduate Biomedical Sciences.
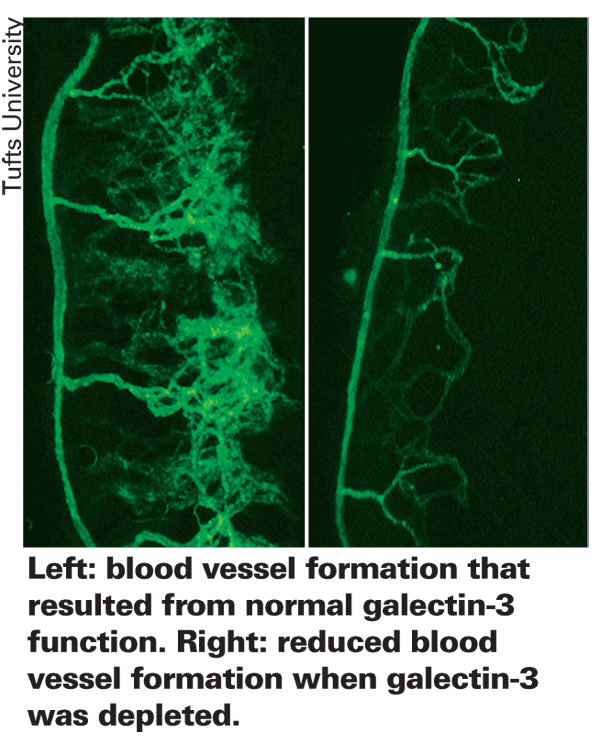 "Our study shows that galectin-3 protein binds to glycans (carbohydrate portions) of specific cell-adhesion proteins, the integrins, to activate the signaling pathways that bring about angiogenesis," said Dr. Panjwani. "This improved understanding may provide a more targeted approach to preventing harmful angiogenesis.
"Our study shows that galectin-3 protein binds to glycans (carbohydrate portions) of specific cell-adhesion proteins, the integrins, to activate the signaling pathways that bring about angiogenesis," said Dr. Panjwani. "This improved understanding may provide a more targeted approach to preventing harmful angiogenesis.
"We found that application of a galectin-3 inhibitor significantly reduced angiogenesis in mice. We also found that preventing galectin-3 from binding with the integrins reduced angiogenesis," said first author Anna Markowska, a PhD student in the biochemistry program at Sackler.
"By deciphering the mechanism of galectin-3 action, we were able to establish more than one therapeutic target. The more we know about how this pathway works, the more options we have for potential treatments," said Dr. Panjwani.
Dr. Panjwani's lab is dedicated to understanding the cell biological and biochemical mechanisms of wound healing and angiogenesis, specifically for the purpose of developing improved treatments for blinding eye diseases. Dr. Panjwani's research is also focused on Acanthamoeba keratitis, a rare and painful parasitic infection of the cornea that can affect contact lens wearers. She is currently working on strategies to protect against the infection and is developing a test that identifies at-risk individuals by sampling their tears.
Iluvien Gets FDA Priority Review For DME Treatment
Alimera Sciences' New Drug Application for Iluvien (fluocinolone acetonide intravitreal insert) has been accepted for filing and granted Priority Review status by the FDA. Iluvien is Alimera's investigational, sustained drug delivery system that releases sub-microgram levels of fluocinolone acetonide for the treatment of diabetic macular edema.
FDA Priority Review status accelerates the standard review time from 10 months to six months. With priority review, Alimera could receive a response from the FDA in the fourth quarter regarding its NDA for Iluvien, which was submitted at the end of June 2010. "If approved, we believe that Iluvien will be the first pharmaceutical in the
Alimera is currently conducting two Phase III pivotal clinical trials (collectively known as the FAME Study) for Iluvien involving 956 patients in sites across the United States, Canada, Europe and India to assess the efficacy and safety of Iluvien with two doses, a high and low dose, for the treatment of DME. The primary efficacy endpoint for the FAME Study is the difference in the percentage of patients whose best corrected visual acuity improved by 15 or more letters from baseline on the ETDRS eye chart at month 24 between the treatment and control groups. The study will conclude later this year with the final patient visits at the three-year data point.



