A Persistent Problem
Why do cases of hydroxychloroquine retinopathy continue to develop, when its pathogenesis has been known since the 1980s? Among several contributing causes, the main one is that too many patients are prescribed toxic daily doses.1 Hydroxychloroquine is stored in lean tissues, and is largely excluded from fat.2 Therefore, dosing of HC should be based on the lean body mass.3 In most people this can be determined from tables of ideal body weight (IBW) based on height. However, in thin, asthenic patients in whom actual body weight is less than ideal, dosing should be based on the actual body weight (ABW), not IBW.4 Although a few cases exist, it’s extremely rare to develop HR if daily dose does not exceed 6.5 mg/kg based on using the lesser of ABW and IBW for the denominator.5
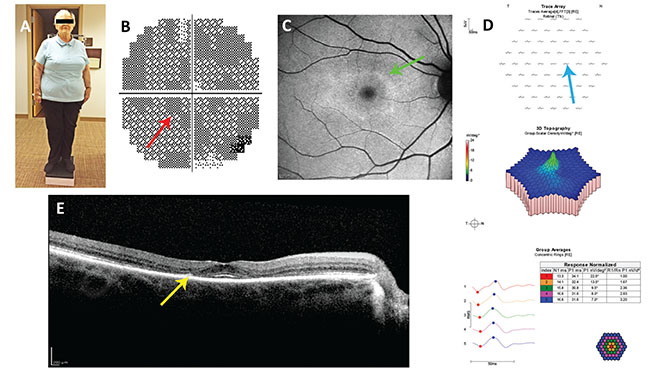 |
| Figure 1. Case report #1. A: Note the somatotype. In the short, obese patient, dosing based on ideal body weight, not actual body weight, is appropriate. B: Paracentral scotomata (red arrow) in her 10-2 visual field test of 2012, missed by her ophthalmologist. C: Annulus of hyperautofluorescence (green arrow) on blue fundus autofluorescence imaging from 2014. D: Reduced paracentral wavelet amplitudes (blue arrow) on multifocal electroretinography. E: Paracentral loss of the ellipsoid line and thinning of the outer nuclear layer (yellow arrow) on spectral domain optical coherence tomography. |
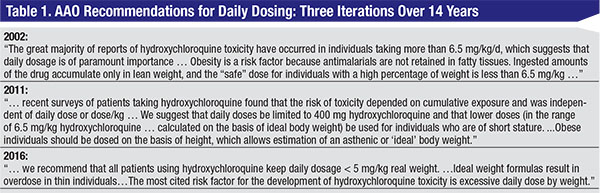
Ophthalmic publications are partially responsible for the confusion that exists regarding the importance of daily dosing in preventing HR. Consider what three consecutive sets of guidelines from the American Academy of Ophthalmology have to say (Table 1): The 2002 guidelines state that daily dose is of “paramount importance,” and advise the use of ABW except in obese patients, in which case IBW is recommended. In 2011, the guidelines flipped, discounting the importance of daily dose unless a patient is obese, in which case dosing by IBW is advised. In 2016, the guidelines flopped, reaffirming the importance of daily dose, but discounting dosing by IBW while stressing dosing by ABW and introducing a different conversion factor.
 |
With such inconsistency, it’s easy to understand how rheumatologists and ophthalmologists might be confused about the importance of the daily dose and how it should be calculated. The 2002 guidelines were potentially dangerous because of their lack of specificity regarding how to use ABW or IBW in the calculation of the daily dose. The 2011 guidelines were potentially dangerous for the person whose ABW is less than IBW (See case 1). The 2016 guidelines are dangerous for the obese person in whom IBW is less than ABW (See case 2).
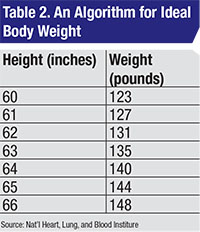
Safe prescribing of hydroxychloroquine isn’t difficult. One takes the lesser of the ABW and IBW based on height.4 The daily dose shouldn’t exceed 6.5 mg/kg/d based on the lesser of ABW and IBW. Which algorithm to use for IBW is controversial, but as a practical concern, probably doesn’t matter. I prefer the National Heart, Lung, and Blood Institute algorithm (Table 2).3
In the following sections, I’ll describe case reports involving HC and explain the dosing issues involved.
Case Report #1
A 74-year-old woman with rheumatoid arthritis was started on hydroxychloroquine 400 mg/d in 2004. She was 5 ft. tall, weighed 198 pounds, and had no renal or liver insufficiency (Figure 1). She was first referred by her rheumatologist for screening for retinopathy in 2012, and had lost the left eye to trauma as a child.
The ophthalmologist who screened her in 2012 found her to have a corrected visual acuity of 20/20 in her right eye and interpreted her 10-2 visual field and multifocal electroretinogram as normal, although they were not. He didn’t recognize her toxic dosing and made no recommendation to change it. She was asked to return in six months but never did.
A different ophthalmologist examined her in 2014. Her corrected visual acuity was 20/20 in the right eye and her fundus examination was normal. A 10-2 VF using a red test object showed progression of an annular scotoma that was present in 2012. A spectral domain optical coherence tomography imaging study of the macula showed loss of the parafoveal ellipsoid zone and thinning of the outer nuclear layer.
This case illustrates the following avoidable, commonly seen traps in the multidisciplinary management of the patient taking HC:
• The rheumatologist prescribed a toxic dose of HC based on the patient’s height and weight. The patient was 5 ft. tall, with an ideal body weight of 123 pounds (55.9 kg, see Table 2) and a maximal daily safe dose of 55.9 x 6.5=363 mg/d of HC. To achieve this using the available pill size of 200 mg, one considers the fact that HC has a long half-life (approximately 40 days1). Thus, one can omit a day of dosing in a week and arrive at a suitable average daily dose. Over a week’s duration, this patient can take up to 7 x 363=2,543 mg of HC safely. If she takes two tablets per day Monday through Saturday and skips HC on Sunday, her weekly total dose will be 2,400 mg which averages out to 2,400/7=343 mg/d, which is less than a toxic dose.
• The patient had no baseline ophthalmic screening examination and wasn’t sent for screening by an ophthalmologist until eight years into therapy. This occurred despite the patient having a high risk for toxicity based on her daily dosing. AAO guidelines state that a baseline exam should be obtained and then, for low-risk patients, yearly examinations should begin five years later. Ophthalmologists have regularly rejected these recommendations.4,7 Rather than continuing to recommend a gap in screening that’s rejected by those tasked with screening for retinopathy, it would be better if the guidelines embraced the practical reality that annual screening is the norm, which in the first years is mainly aimed at detecting and correcting toxic daily dosing.
• The doctor failed to recognize and correct the toxic dosing of HC. Toxic dosing is consistently found in 12.8 to 74.7 percent of patients taking HC.4,8-10 Correcting toxic daily dosing is the most common action taken by the screening ophthalmologist, and is the main reason to screen patients taking HC. Where safe dosing is assumed, as in Great Britain, screening isn’t recommended because the rarity of HR in these circumstances makes it a wasteful use of scarce resources.11
• The first ophthalmologist failed to recognize the evidence of toxicity on the initial 10-2 VF. The diagnosis of HR is based on ancillary testing: 10-2 VF; SD-OCT; mfERG; and fundus autofluorescence imaging. Ophthalmologists who screen for HR have an obligation to understand what constitutes a positive test, what the potential artifacts are, and how to use multiple modalities to raise clinical confidence in a diagnosis. The topic has been thoroughly explored in multiple publications.1,12,13 Failure to properly interpret patient testing has formed the basis of large malpractice settlements in missed cases of HR.
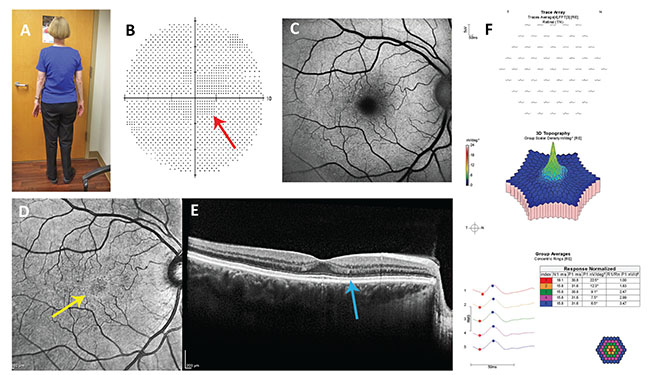 |
| Figure 2. Case report #2: A: Note the somatotype. In the asthenic patient, dosing based on actual body weight, not ideal body weight, is appropriate. B: Nonreproducible central scotoma (red arrow) in her 10-2 visual field test isn’t typical of hydroxychloroquine retinopathy. C: Blue fundus autofluorescence imaging is normal. D: Near infrared reflectance imaging shows a hyperreflective annulus (yellow arrow) which has low sensitivity and specificity for hydroxychloroquine retinopathy. E: The reflectivity of the ellipsoid line in the parafovea is slightly less than in the fovea, a potentially early sign of retinopathy. F: Multifocal electroretinography is normal. |
Case Report #2
In 2015, an ophthalmologist examined a 63-year-old woman with rheumatoid arthritis who had been taking HC 400 mg/d since 1990 (25 years, 3,650 g cumulative dose). She was 5 ft. 3 in. tall and weighed 112 pounds. She had no renal or liver disease. Her corrected visual acuity was 20/20 and her fundus examination was normal bilaterally. Her 10-2 visual field performed with a red test object showed a nonreproducible relative central scotoma (Figure 2). Her fundus autofluorescence imaging was normal. Her near infrared reflectance imaging showed a faint hyperreflective ring, which has inconsistently been associated with early HR.14 The SD-OCT showed a slightly less hyperreflective ellipsoid line and a suggestion of outer nuclear layer thinning. Multifocal electroretinography was normal.
Because there was no definite retinopathy, she was cleared for continued use of HC, but her toxic dosing was corrected. The lesser of her ABW and IBW was her ABW, which was 112 pounds. Therefore, the upper limit of her safe daily dose was 330 mg. It was recommended that she take no more than 200 mg/d for the next six months at which time repeat ancillary testing was recommended to determine if there was a signal for early retinopathy given her large cumulative dose.
Rheumatologic publications also contribute to confusion on dosing. In an April online article from the rheumatology website RheumNow.com, the author writes, “The known risk factors for the development of ocular deposition include duration of therapy, cumulative dose and renal function.”15 She doesn’t mention the most important, and only modifiable, risk factor—daily dosing. She goes on to write, “The American Academy of Ophthalmology (AAO) [citing the 2011 guidelines] advises weight-based dosing of 6.5 mg/kg, with an upper limit of 400 mg/day. Exceptions are individuals of short stature and obese patients, for whom the AAO advises calculating dosage based on ideal body weight for height.” This is not what the quoted reference states, however. Instead, it states, “The prior recommendation [the 2002 guidelines] emphasized dosing by weight. However, most patients are routinely given 400 mg of HCQ daily. ...This dose is now considered acceptable, except for individuals of short stature, for whom the dose should be determined on the basis of ideal body weight to avoid overdosage.”16
The author continues, “The sensitivity and specificity of these tests are not yet known for hydroxychloroquine-related retinal toxicity. There is a high rate of baseline abnormalities, in particular in those who are elderly or have comorbid disease, which make the changes challenging to interpret. Another issue is that systemic lupus erythematosus itself associates with the presence of retinal abnormalities, adding further to the complexity of deciphering these tests.” These are incorrect statements. The relative sensitivity and specificity of the tests are known (mfERG is most sensitive, 10-2 VF next, and SD-OCT least; SD-OCT is most specific, 10-2 VF is next, and mfERG is least).17 The rate of baseline abnormalities is high for 10-2 VF testing, but not for SD-OCT or mfERG. SLE rarely affects any of the tests.
 |
Finally, the author advocates dosing based on 6.5 mg/kg/actual body weight. This is a dangerous recommendation for the short, obese patient as exemplified in Case Report 1.
In a rheumatologic review of HR the authors wrote, “A French study showed that whole-blood HC concentration >1,000 ng/ml reduced risk of lupus flares. Measurement of HC blood levels may suggest that some patients require >6.5 mg/kg/day to achieve the recommended level. These patients should not be at increased risk of toxicity, providing levels are monitored, but it may be important to monitor cumulative dose as well when assessing risk of ocular toxicity.”18 The statement erroneously suggests that there is a known relationship between HC blood levels and HR, but this is not the case.
If prescribing internists and screening ophthalmologists put a clearer understanding of correct daily dosing into practice, the prevalence of HR would fall. In most cases, the condition is iatrogenic and can be avoided. Daily dosing is the only modifiable risk factor and deserves the greatest attention in screening visits, especially in the first five years of use of the drug, before the irreversible, cumulative load has built up and raises the retinopathy risk. The recommendation that low-risk cases have screening omitted for five years after a baseline examination has been widely rejected by those who do the screening, and is probably misguided given the high prevalence of toxic dosing. Detection and correction of toxic dosing is of greatest value for the prevention of retinopathy in the early years of taking HC. The extremely rare cases that develop retinopathy despite safe dosing are more likely to be detected when a more sensitive and more specific ancillary test, for example, 10-2 VF and SD-OCT, are used together and when interpretation is competently done.
Dr. Browning is a retina specialist at Charlotte Eye, Ear, Nose, and Throat Associates. Contact Dr. Browning at 6035 Fairview Rd, Charlotte, NC 28210. He can be reached at (704)-295-3180, or dbrowning@ceenta.com.
1. Browning DJ. Hydroxychloroquine and chloroquine retinopathy. New York: Springer, 2014.
2. McChesney EW, Banks WF Jr, Fabian RJ. Tissue distribution of chloroquine, hydroxychloroquine, and desethylchloroquine in the rat. Toxic App Pharm 1967;10:501-513.
3. Browning DJ, Lee C, Rotberg D. The impact of different algorithms for ideal body weight on screening for hydroxychloroquine retinopathy in women. Clinical Ophthalmology 2014;8:1401.
4. Browning DJ. Impact of the revised American Academy of Ophthalmology guidelines regarding hydroxychloroquine screening on actual practice. Am J Ophthalmol 2013;155:418..
5. Falcone PM, Paolini L, Lou PL. Hydroxychloroquine toxicity despite normal dose therapy. Ann Ophthalmol 1993;25:385-388.
6. Browning DJ. Reply to Defining Ideal Body Weight. Am J Ophthalmol 2002;134:935-936.
7. Blomquist PH, Chundru RK. Screening for hydroxychloroquine toxicity by Texas ophthalmologists. J Rheumatol 2002;29:1665.
8. Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res 2010;62:775-784.
9. Cukras C, Huynh N, vitale S, Wong WT, Ferris III FL, Sieving PA. Subjective and objective screening tests for hydroxychloroquine toxicity. Ophthalmology 2015;122:356-366.
10. Lee MG, Kim SJ, Ham DI, Kang SW, Kee C, Lee J, Cha HS, Koh EM. Macular retinal ganglion cell-inner plexiform layer thickness in patients on hydroxychloroquine therapy. Invest Ophthalmol Vis Sci 2015;56:396-402.
11. FielderA, Graham E, Jones S, et al. Royal college of ophthalmologists guidelines: Ocular toxicity and hydroxychloroquine. Eye 1998;12:907-909.
12. Marmor MF, Melles RB. Disparity between visual fields and optical coherence tomography in hydroxychloroquine retinopathy. Ophthalmology 2014;121:1257-1262.
13. Mititelu M, Wong BJ, Brenner M, et al. Progression of hydroxychloroquine toxic effects after drug therapy cessation. New evidence from multimodal imaging. Arch Ophthalmol 2013;131:1187-1197.
14. Wong KL, Pautler SE, Browning DJ. Near-infrared reflectance bull’s eye maculopathy as an early indicator of hydroxychloroquine toxicity. Clin Ophthalmol 2015; 9:521-5.
15. Petrie M, Durcan L. “The role of hydroxychloroquine blood levels in SLE” found at http://rheumnow.com/blog/role-hydroxychloroquine-blood-levels-sle#.VvaKbIfbwMQ.mailto. Accessed 28 September 2016.
16. Marmor MF, Kellner U, Lai TYY, Lyons JS, Mieler WF. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011;118:415.
17. Browning DJ, Lee C. The relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clinical Ophthalmology 2014;8:1389-1399.
18. Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinopathy. Rheumatology (Oxford) 2016;55:6:957-67.



