In recent years, a new class of drugs has caused a revolution in the treatment of age-related macular degeneration. These agents interfere with the action of vascular endothelial growth factor, or VEGF, which triggers the growth of blood vessels. During normal growth processes, such as embryonic states, VEGF is a key regulator of angiogenesis. However, if it goes out of balance it can trigger the growth of extra, unwanted vessels, or neovascularization. This can directly cause damage, as it does in diseases like AMD or neovascular glaucoma, or contribute to another disease process, as it does by aiding the growth of tumors in some forms of cancer. (Anti-VEGF treatment was first developed for tumor therapy, especially in colon cancer where it can cause tumors to shrink.)
The connection between neovascular glaucoma and VEGF compounds was first made because of a 1994 article in the New England Journal of Medicine.1 A study found an increased concentration of vascular endothelial growth factor, or VEGF, in both the vitreous and aqueous in patients with diabetes—especially proliferative diabetic retinopathy with its associated neovascularization. Panretinal photocoagulation (PRP), a traditional treatment that destroys ischemic retinal tissue, was found to decrease the concentration of VEGF by about 75 percent.
Neovascularity and PRP
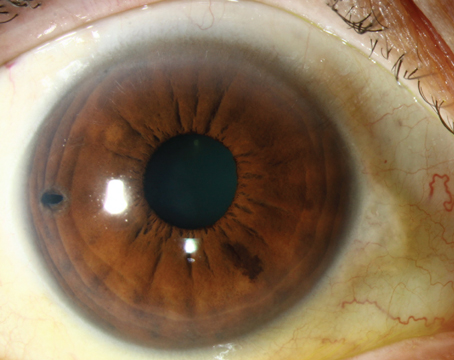
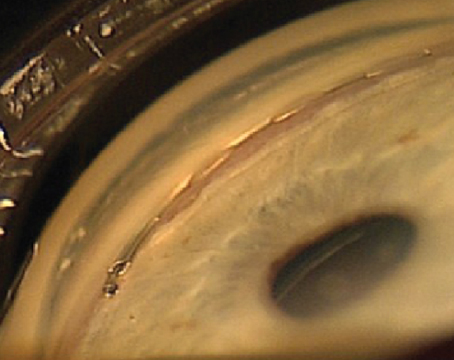
In the eye, VEGF is produced in the posterior chamber, but it does filter into the anterior chamber. As a result, an eye may exhibit "close" neovascularization—adjacent to the ischemic or vascular retina—or "distant" neovascularization in the iris or trabecular meshwork. If the concentration of VEGF becomes very high, the neovascularization can even extend to the anterior lens surface.
As a result, in many forms of ocular disease, neovascularity is part of the end stage. That's certainly the case in neovascular glaucoma. Vessels grow over the TM and iris, causing fibrovascular contraction, leading to synechiae and closure of the TM, or directly causing destruction of the TM. Either way, open channels become closed, interfering with the outflow of aqueous, and pressure builds inside the eye. For that reason, any disease that involves ocular ischemia may lead to neovascularization and eventual glaucoma.
One traditional way of treating this type of neovascularization is with panretinal photocoagulation. This approach has two serious problems, however. First, PRP destroys the peripheral retina. Thus, if a patient suffers from central retinal vein occlusion, the disease has already destroyed central vision; now the treatment destroys much of what's left. Second, the desired effect—a reduction in neovascularization—takes time to occur, so the eye doesn't get immediate relief.
For that reason, the classic approach to treating CRVO is monitoring the patient in the hope that neovascularization doesn't develop. Unfortunately, CRVO is sometimes called "90-day glaucoma," because within 90 days a lot of these patients develop neovascularization in the iris and the angle. If you don't catch it early, the patient can develop glaucoma severe enough to lose the eye.
Using Avastin
I first became interested in the idea of using Avastin (bevacizumab, Genentech) to treat neovascular glaucoma because I work closely with retina specialists who use it to treat symptoms of age-related macular degeneration. The first time we tried it as a means of treating neovascular glaucoma, the result was much better than we had expected; regression of the vessels occurred rapidly. Since then, we've used it more and more for neovascularization-related problems in the eye.
The effectiveness of the treatment seems to depend both on the stage of the disease and the individual patient. In my experience, severe disease may require multiple injections to achieve significant regression; some cases even require the combination of PRP and Avastin injection. On the other hand, one patient who presented with mild disease involving one area of neovascularization in the angle received a single injection and has had no further neovascularization for nine months. (For more details, see "A Case History," below.)
Of course, using a compound like Avastin to regress neovascularization is a great option, but if you don't deal with the underlying disease more VEGF will eventually be produced, even in the best of circumstances. In diabetic retinopathy, for example, the patient needs to control his blood sugar level or the problem will eventually recur. Also, supplemental PRP is still helpful in most cases.

Another anti-VEGF option, of course, is Lucentis (ranibizumab, Genentech), which is chemically similar to Avastin. However, the cost difference is significant; an injection of Lucentis can cost close to $2,000, compared to an injection of Avastin which costs at most $100. Given the cost difference, this is a significant advantage for use of Avastin. (Neither drug is FDA-approved for treating neovascular glaucoma.)
Avastin and Surgery
In most cases, when a retina specialist sees neovascularization in the iris or angle, he or she will consult with a glaucoma specialist to help manage the potential glaucoma. However, we sometimes do see a patient who has already reached the stage of severe neovascularization in the iris and angle with very elevated pressure and synechial closure of the angle—stage three neovascular glaucoma. If your first contact with the patient occurs at this point, even using Avastin to trigger regression of the neovascularization may not prevent the need for glaucoma surgery; a lot of the trabecular meshwork and the angle has already been damaged by overgrowth of the vessels and peripheral anterior synechiae formation.
Nevertheless, anti-VEGF compounds may be helpful even at this stage. A patient in this condition requires emergency surgery, but the eye is severely inflamed with an elevated IOP; when you decompress an eye in this situation you risk causing the excess blood vessels to bleed profusely, causing vitreous hemorrhage, suprachoroidial hemorrhage and possible loss of the eye.
We have found, however, that an injection of Avastin a day or two before the surgery—or even during surgery—causes a noticeable decrease in the bleeding. In this way, the drug may save the eye from a potentially blinding complication of the glaucoma surgery.
Related Issues
As a new option for glaucoma treatment, use of anti-VEGF drugs raises a number of considerations:
• Intravitreal injections. Many general ophthalmologists—and glaucoma specialists—are apprehensive about giving intravitreal injections. A less imposing alternative is intracameral injection. I used this approach myself in one case in which the patient's lens had become vascularized and the patient had no-light-perception vision. The intracameral injection worked; the vessels disappeared.
The problem with this approach is that we don't know much about its safety. Few studies of intracameral anti-VEGF have been done, and we don't know what level might induce toxicity. The existing case report certainly suggests that one dose is not toxic, but more studies need to be done to confirm this. In the meantime, if repeat injections are necessary the surgeon giving them is moving into uncharted territory.
In contrast, we know much more about intravitreal injection, thanks in part to the extensive studies of Lucentis and the literature on Avastin. The data have shown that intravitreal injection of Lucentis once a month for several months is safe for the retina and anterior segment, at least in the short run.
• How much, how often? Because this approach is so new, we're still in the early stages of learning. We're currently collecting patient data, which we plan to review to get a better idea of how many injections are necessary to achieve regression of neovascularization (on average), and how long it takes for the effect of Avastin to go away if we don't resort to PRP.
Also, safety is always a concern when using a drug for a new purpose. We know that, so far, Avastin injection has been safe in the eye. However, we also know that it's not entirely safe when used systemically for colon cancer. Although it causes regression and shrinkage of colon cancer tumors, it has been associated with gastrointestinal bleeding and vascular strokes.
Up to now we haven't had a problem with ocular injection, but we can't assume that it is 100-percent safe until more research has been done.
• Retinopathy of prematurity. Researchers in
• Preventing bleb failure. Recently, some research has focused on the possibility of using an anti-VEGF compound like Avastin to help prevent bleb failure following trabeculectomy. (Neovascularization, of course, is not the only cause of bleb failure; inflammatory products can cause scarring and fibrosis.) This research is still in its early stages, and because bleb problems happen over time, finding out whether Avastin or another drug can serve this purpose will probably require a multicenter clinical trial running for at least a year.
If it turns out that a drug like Avastin or Lucentis is more effective in decreasing bleb failure and safer than mitomycin-C, most surgeons would probably switch to using it.
A Promising Approach
Avastin has been very helpful in our glaucoma practice. By using it early on in cases of neovascularization, we've been able to prevent damage to the eye and the need for glaucoma surgery. And by using it before surgery in more serious cases, we've been able to prevent bleeding and avoid surgical complications. That's definitely a step in the right direction.
Dr. Al-Aswad is assistant professor of clinical ophthalmology at the Columbia University College of Physicians and Surgeons, Edward S. Harkness Eye Institute.
1. Aiello LP, Avery RL, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;1;331:22:1480-7.