Here, surgeons familiar with both systems talk about the current status of the HOLOS system, the ways in which ORA and HOLOS differ and why they believe surgeons should seriously consider adding this type of technology to their cataract surgery armamentarium.
HOLOS: A New Formula
Barry Linder, MD, chief medical officer at Clarity Medical Systems, explains that the HOLOS IntraOp system is currently in limited release in the United States. Its capabilities right now include monitoring real-time refraction throughout surgery, confirmation of hitting a target refraction and the ability to neutralize cylinder in pseudophakia with a toric lens and/or titrate limbal relaxing incisions at any point in the surgery. One of the key functions of intraoperative aberrometry—using the aphakic refraction to predict a spherical lens power—is currently being finalized and should become part of the system by late summer.
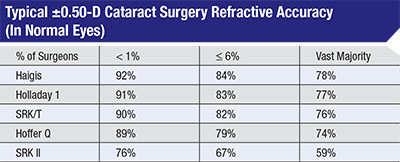 |
Experts say that many surgeons don’t carefully evaluate their outcomes, often assuming their results are better than they actually are. Warren Hill, MD, assembled this data from more than 260,000 optical biometry cases submitted for Haigis formula optimization from 2004 to 2015. Less than 1 percent of surgeons consistently got 90 percent of patients to within 0.5 D of target; most surgeons only had 59 to 78 percent fall within that range. |
“We’re still collecting the data that our external consultants—including Doug Koch, Warren Hill and Graham Barrett—are using to finalize the development of our IOL algorithm,” explains Dr. Linder. “Dr. Barrett is doing much of the heavy lifting, with the Barrett Universal II and Rx formulas as the starting point. The resulting formula will be called the HOLOS-Barrett IOL formula. We’re making terrific progress; we have the basic outline of the algorithm and we’re integrating it into our software. The new version of the HOLOS software will also include new database management tools, such as a cloud-based physician portal for preoperative data input and connectivity to the HOLOS device in the OR via the cloud.”
Warren E. Hill, MD, FACS, medical director of East Valley Ophthalmology in Mesa, Ariz., is one of the experts helping to collect data and finalize the formula that will become part of the HOLOS system in the next few months. “The formula will take the vergence of the aphakic eye and convert it into an IOL power for the doctor to use at the time of surgery,” he explains. He notes that this type of formula is important because the pseudophakic spherical power measurement taken after lens implantation may not be as accurate as a formula’s prediction. “The pseudophakic confirmation is good to have,” he says, “but most surgeons who use intraoperative aberrometry depend more on the aphakic reading and the IOL power recommendation made by the instrument.
“The issue here is that the power of the lens inside the eye is relative and not absolute,” he continues. “A 21-D lens is only 21 D at one specific distance from the cornea. When you’ve just put the lens inside the eye, its position has not yet been finalized by capsular bag contraction. In the pseudophakic state this technology is outstanding for seeing whether the corneal astigmatism has been corrected, but it may be less useful as an immediate means to check the spherical power. On the other hand, surgeons often change their original lens choice up or down 0.5 D based on the aphakic measurements and formula’s lens suggestion.”
HOLOS Features
In addition to implementing the new formula, the designers of the HOLOS system are working to incorporate features that will help it compete with the ORA. Those features include:
• A new aberrometer. “One advantage of the HOLOS instrument is that its aberrometer is a technologically advanced device that was designed specifically for this purpose,” notes Dr. Hill. “The ORA uses a Talbot moiré aberrometer, technology from the 1980s that was developed for a different purpose. The HOLOS aberrometer is very fast and very accurate, and the data is continuously filtered through measurement qualifiers. That means that before a measurement is displayed on the screen it has to pass muster, so to speak. As a result, the HOLOS aberrometer is accurate within 0.25 D at the corneal plane, with a 40-D dynamic range. And because the data is continuously displayed, a separate person is not needed in the OR to operate the system.”
• Data presentation. Dr. Linder says the forthcoming upgrade will include significant updates to the graphical user interface on the device, for use in the OR. “The HOLOS system will present the data in a unique way,” he says. “Current users of intraoperative aberrometry capture several ‘snapshot’ readings before making an IOL power selection, in order to feel confident that the measurements are accurate. This can take several minutes to accomplish. The HOLOS system constantly takes about 90 readings per second and qualifies each one so that it makes its IOL calculations using only readings that are qualified. And instead of showing the result of snapshots taken by the surgeon, the HOLOS will display a frequency histogram that shows, in real time, what percentage of the time a particular IOL power is recommended by the formula. (See sample screenshot, facing page.) It will be easy to see which IOL power is supported by most of the readings. This is a very different way to manage this information. It’s intuitive, easy to use and much faster than stopping and taking snapshots of periodic readings.”
• No need to refocus. Dr. Linder notes that the instrument’s focus doesn’t need to be changed as the microscope is adjusted. “The ORA system requires periodic refocusing,” he says. “With HOLOS, the focal point is kept at the iris plane or wherever the surgeon is operating. The data is always being generated and qualified, and the surgeon doesn’t have to re-adjust the scope to get the qualified readings. It’s one less thing for the surgeon to think about.”
Keith Liang, MD, medical director of the Sacramento Eye Surgery Center in Calif., says he’s been using the HOLOS system since Clarity began developing it; he’s currently providing data to help with the development of the new IOL power formula that will soon be incorporated into the system. He says he likes not having to refocus the HOLOS when the microscope is adjusted. “You don’t have to change the focus or turn off the microscope light and elevate the system to a certain height above the cornea to obtain a reading, like you do with ORA,” he says. “With the HOLOS system, the focus corresponds to your focus in the microscope. That helps your OR efficiency.”
• Improved light visibility through the optics. Dr. Linder says the optics in the HOLOS system are designed to take less light away from the optical path in the microscope than the ORA does. Dr. Liang says he has noticed the difference. “Both instruments go under the microscope, but with HOLOS the image coming through the oculars remains bright,” he says. “When you put the ORA system underneath the microscope, it reduces the light coming through the oculars noticeably. You can counteract that by increasing the intensity of the light, but sometimes that’s uncomfortable for the patient. For the surgeon it’s very ergonomic to keep the lighting the same.”
Advantages of ORA
P. Dee G. Stephenson, MD, current president of the American College of Eye Surgeons and an associate professor of ophthalmology at the University of South Florida College of Medicine in Tampa, has been using the ORA system for many years; she was the first commercial user of the system in the United States. “ORA has 500,000 cases in its AnalyzOR databank,” she notes. “There are many different lens model outcomes that have been optimized in ORA, so it will be a long time before HOLOS has the experience that ORA has. ORA paved the way in intraoperative aberrometry, and every upgrade they’ve made has been an improvement.
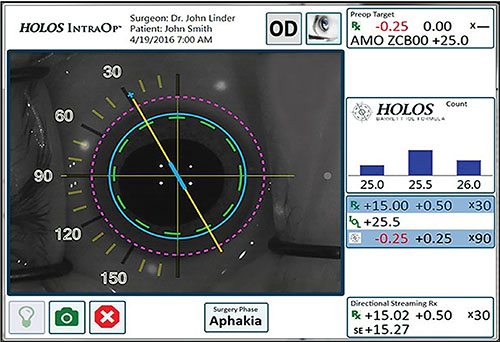 |
The HOLOS IntraOp system screen displays qualitative and quantitative data in real time. Readings are taken about 90 times per second; only readings that reach acceptable parameters are incorporated into the displayed data. The large image on the left shows refractive information as a dynamic qualitative display superimposed over a live video of the patient’s eye. The box resembling a bar graph (right)shows the lens power recommendation calculated by the HOLOS-Barrett IOL formula, displayed as a proprietary histogram. The box below that displays the qualified, quantitative refractive data, including sphere, cylinder, axis, lens diopter and expected postop refraction. |
“There are some great minds working on the HOLOS system,” she acknowledges, “but when you come out of the gate, you’re not going to be perfect. It’s taken ORA more than seven years to get where it is, and it works amazingly well. In particular, it has become very accurate with most post-refractive patients, and it’s getting better with post-RK and post-hyperopic LASIK patients.”
Dr. Linder notes that while the HOLOS system will not initially have the advantage of incorporating data from thousands of cases, as the ORA system currently does, it won’t be starting from zero. “Our formula will be based upon the Barrett Universal II and Rx formulas,” he points out. “These formulas are well-understood and validated; they are considered to be some of the most accurate in the world. So we’re not starting from scratch; we’re building upon very well-accepted and validated IOL algorithms.
“In addition, the team that’s developing this has an extraordinary amount of experience,” he continues. “For example, Warren Hill has more than a quarter million cases that can be used to adjust constants based on factors such as design, materials, haptic/optic junction angulation and stability in the eye. So although we won’t have a huge database of cases at the outset, we have the foundation of a validated formula developed by surgeons with some of the most advanced knowledge available regarding how to optimize constants for the variety of IOLs that a surgeon might use.”
Dr. Stephenson agrees that the two Barrett formulas used as the basis for the HOLOS predictive formula are well-established and accepted, but notes that the ORA uses multiple leading formulas, including the SRK/T and Holladay I. “The ORA system uses a modified version of the refractive vergence formula, which incorporates the measured aphakic spherical equivalent,” she says. “It also analyzes regression coefficients—every lens model has a unique set of them—and the surgeon’s factor. Those data are refined and optimized globally and quarterly, incorporating the postoperative refractive data you enter. When you use it for the very first time, your information will be compared to global data. Over time, the system personalizes your own surgeon factor.”
Those working on the HOLOS system often point out that it captures data in real time, citing this as an advantage over the ORA. Dr. Stephenson takes issue with that. “I know HOLOS says it’s real-time, but so is ORA,” she says. “There’s a streaming refraction on the top of the screen, and as you move the eye, that changes. When you take a reading, you’re capturing one moment in time, but you see the changes occurring in real time.”
Dr. Linder acknowledges that ORA users also see refractive data changing in real time during surgery, but still says there are differences. “The frequency of the data collection and how it’s processed and displayed is significantly different,” he says. “Our data is being processed and qualified 90 times a second, and all of the qualified data points will be fed through the predictive algorithm. The onscreen histogram display will show which prediction is being supported the most by the ongoing data, which could include hundreds or thousands of measurements, depending on how many seconds the surgeon chooses to hold the eye under the device during aphakia. So the process will be very fast and intuitive, leading to the surgeon having a high level of confidence in the prediction.”
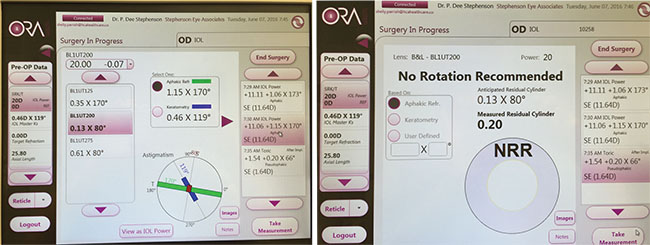 |
The ORA system currently incorporates data from 500,000 cases, with many different lens models optimized. Surgeons using the system report that it has become very accurate with post-refractive eyes. |
Is the Tech Worth the Cost?
As is often the case, adding new technology like intraoperative aberrometry can be an expensive proposition. Nevertheless, many surgeons see it as a worthwhile investment. “All surgeons pride themselves on great outcomes,” Dr. Stephenson points out. “If you ask your colleagues about their outcomes, most people will say they’re within 0.5 D of their intended target. But if you look at the real world, only between 70 and 80 percent of surgeons actually are.1-3 In reality, most surgeons only guesstimate what their surgically induced astigmatism is in each eye. They never actually calculate it. The problem is, to get great outcomes, you have to know those things.”
Dr. Stephenson says that she’s had great results using intraoperative aberrometry. “I just published data from 150 cases that are two years out, all done using ORA,” she says. “About 92 percent of these patients have 0.5 D or less of residual cylinder, and about 86 percent have 0.25 D or less. When addressing astigmatism, I don’t even mark my patients any longer. I go by what ORA tells me.”
Dr. Hill agrees that doctors who are skeptical of the value of this kind of technology usually aren’t tracking their outcomes. “Very few people do, and most surgeons think they’re getting much better results than they actually are,” he says. “The vast majority of ophthalmologists have ±0.50-D accuracy in the mid- to high-70 percent range. Intraoperative aberrometry will typically move most surgeons into the mid- to high-80 percent range. Of course, because we haven’t finalized the formula for HOLOS, this is ORA data.”
Dr. Liang says that whether or not intraoperative aberrometry is worth the cost for a given practice depends on several factors. “For one thing, the more surgeons you have in your practice or surgery center, the lower the cost will be,” he says. “It also depends on how many premium lenses you’re doing, because for those patients it’s especially important to deliver what you’re promising. Furthermore, the more confident you are that you can deliver on your promised outcome, the more you’ll offer the premium lenses.
“For example,” he continues, “at the last Ophthalmology Innovation Summit meeting a survey suggested that only 7 or 8 percent of IOLs being implanted were toric IOLs; meanwhile, more than 65 percent of patients getting cataract surgery have significant astigmatism that could be corrected. Another poll found that most doctors don’t believe that a toric lens rotation of 10 degrees or less is significant in terms of patient satisfaction. That’s a dangerous assumption to make. In any case, intraoperative aberrometry definitely improves toric lens power selection and alignment, although it takes a little extra time in the OR. I see a lot of surgeons who make a quick mark and use that to guide the alignment. This is a much more accurate way to proceed. With intraoperative aberrometry, you can be very confident of your correction.”
| Putting the Surgeon in Charge |
| Keith Liang, MD, medical director of the Sacramento Eye Surgery Center, notes that one of the things that makes data produced by intraoperative aberrometry different from preoperative data and calculations is that intraoperative aberrometry is entirely measured by the surgeon. “When you’re taking the measurement in the OR, it’s you, the surgeon, who dictates the accuracy of the reading,” he says. “In your preoperative calculations, you’re rarely the person running the instruments and taking the measurements. Obviously you oversee the preoperative measurement process and train your staff well, and there are things that can help you determine whether the preoperative readings are good or not. But you can’t take the readings yourself in a busy clinic. In contrast, you have complete control when you take a reading with intraoperative aberrometry.” P. Dee G. Stephenson, MD, current president of the American College of Eye Surgeons and an associate professor of ophthalmology at the University of South Florida College of Medicine in Tampa, a long-time user of the ORA system, notes that if you want to get accurate information using intraoperative aberrometry, managing the details matters. “You have to follow a cookbook recipe to use this technology, but if you follow it, it really works,” she says. “You have to keep the cornea clear. You have to get all of the viscoelastic out of the cul-de-sac. You can’t have pressure on the eye from the lid speculum. You can’t just guesstimate the intraocular pressure by feeling the eye; you have to measure it with the Barraquer tonometer, and it has to be about 20 mmHg. You can do final readings with either BSS or viscoelastic in the eye, but it can only be one type of viscoelastic; you can’t have two kinds in the eye when you take the reading.” Dr. Liang notes that this is all on the surgeon. “When you’re in the OR with the HOLOS or ORA, you can’t blame anyone else for not lining up the eye, not getting the pressure right or not irrigating the surface,” he says. “It’s all up to you. Maybe your preoperative calculations are great and the system will confirm them time after time. Maybe, after analysis, you’ll find that the intraoperative aberrometry data is better, and you can then work to find out why that’s the case and improve your preoperative measurements or calculations. Either way, you should end up generating better outcomes.” —CK |
Dr. Stephenson adds that the technology is most worthwhile if you take maximum advantage of it. “The holy grail is a great IOL power formula, but I find that ORA is excellent at picking the power of the implant,” she says. “I use it on 99 percent of my patients, and I change my power choice about 52 percent of the time based on what ORA tells me. I have state-of-the-art equipment for making preoperative measurements, and the ORA agrees with the predictions based on that data much of the time. If my information going into the OR is confirmed by ORA, that’s great. But if I have to hedge one way or the other, I always go with ORA.”
Dr. Hill says he plans to use intraoperative aberrometry with every cataract patient. “Less than 1 percent of surgeons are at ±0.50-D accuracy for 92 percent or more of their patients,” he says. “Only 6 percent achieve this accuracy for 84 percent of their patients. Everyone else is at about 78 percent. That’s the reality. Certainly, if you’re putting in a multifocal or toric lens, something you charge the patient extra for, surgeons in general need to up their game. HOLOS will be one way to do this.”
“If 90 percent of the surgeons using this technology get within 0.5 D of their target, why wouldn’t you want to be one of those surgeons?” asks Dr. Stephenson. “Today, the world is full of post-LASIK and post-refractive surgery patients. They want the same ‘wow factor’ that they got with LASIK. The only way to do that is with a technology like intraoperative aberrometry.”
Just the Beginning
“Intraoperative aberrometry technology is still in its early stages,” notes Dr. Liang. “Right now, we’re trying to figure out exactly how to use this technology. If you’re straddling between two powers that are close, the intraoperative aberrometry reading can push you towards one or the other. But if the aberrometer gives you a very different number than your preoperative calculations, then you have to use your brain. Are things lined up? Is the eye not staring at the fixation light? Is the surface too dry? Did I not have the correct pressure inside the eye? The real-time feedback helps the surgeon decide whether an unexpected reading is worth taking seriously, because changes in the readings are often associated with real-time actions such as pushing on the lid speculum.
“When topography first became available,” he adds, “some people said, ‘I don’t need topography to do cataract surgery or LASIK.’ But as we learned to use the systems and the technology improved, its importance became obvious. Today, no one would say that they can do cataract surgery without topography. I think intraoperative aberrometry will follow the same path. Someday, hopefully, this will be the standard of care.”
Dr. Liang adds that no matter how good intraoperative aberrometry gets, it will never replace good preoperative measurements. “I would never tell someone that because you have an ORA or HOLOS you can turn off your brain and skip the detailed preoperative measurements,” he says. “What intraoperative aberrometry adds is another piece of information. If we want to do refractive cataract surgery we have to at least have a goal of coming close to LASIK outcomes. Intraoperative aberrometry will help us move in that direction.”
Dr. Linder says Clarity Medical Systems hopes to obtain the CE Mark for HOLOS later his year, in time to launch the upgraded version of the system that will incorporate the new IOL formula, at the European Society of Cataract and Refractive Surgery meeting. “After that,” he says, “we’ll continue to refine the algorithm as data comes in and continue to refine the physician portal so that the surgeon can get reports on his outcomes in comparison to baseline data and the overall database. We’ll be able to provide customized physician factors based on things like the surgeon’s style of wound and the location of the incision. We anticipate that this level of customization will help surgeons further improve their outcomes.”
Dr. Stephenson says she hopes the HOLOS system will eventually be just as good as she finds the ORA to be. “This is just the tip of the iceberg,” she says. “The great thing about technology is that it promotes competition from many companies and challenges the technology that’s already on the market to become even better. There are some brilliant engineers behind ORA, so I can’t wait to see what will come next.
“If I had to pick one instrument that I could use in the OR that would make my patients happier and improve their outcomes, it would be ORA,” she adds. “I wouldn’t choose a femtosecond cataract surgery system, because if your preoperative measurements are wrong, it doesn’t matter how precise your surgery is. Today, things like getting your patients’ residual astigmatism below 0.5 D are crucial if you want to have happy patients. To achieve that, you need technology like this.” REVIEW
Dr. Stephenson was previously a consultant for WaveTec. Drs. Liang and Hill are consultants for HOLOS.
1. Gale RP, Saldana M, Johnston RL, et al. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (Lond) 2009;23:1:149-152.
2. Hahn U, Krummenauer F, Kölbl B, et al. Determination of valid benchmarks for outcome indicators for cataract surgery: A multicenter, prospective cohort trial. Ophthalmology 2011;118:11:2115-2112.
3. Behndig A, Montan P, Stenevi U, et al. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J Cataract Refract Surg 2012;38:7:1181-1186.