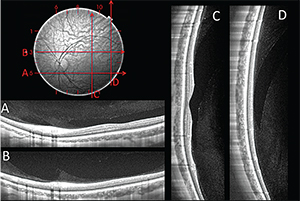
 |
| Figure 1. A horizontal SS-OCT scan at the level of the optic nerve shows the premacular bursa and the beginning of Cloquet’s canal separated by what Jan Worst called the “septum interpapillomaculare” (A). The premacular bursa continues superiorly on this vertical scan through fixation obtained on upgaze (B). The superior extension of the premacular bursa and Cloquet’s canal are seen to fuse at some distance from the optic nerve in this horizontal scan (C). |
The Pioneers’ Vision
Two pioneers of vitreous anatomy, Jan Worst and Shoji Kishi, used complementary methods to explore the vitreous spaces, the former by injecting the liquid spaces with ink,1 the latter by staining the formed vitreous with fluorescein.2 Their findings were also complementary, with—among other differences—Prof. Worst stating that the “premacular bursa” was a liquid space overlying the macula, and connecting to a retrociliary system of other liquid-filled spaces he termed “cisterns,”1,3 while Prof. Kishi claimed that the “posterior precortical vitreous pocket”—arguably the same space—was confined, and boat-shaped.2 Prof. Kishi accepted connections to other liquid spaces only later in life in the context of vitreous degeneration. He and his team went on to utilize in vivo imaging techniques, namely optical coherence tomography, to characterize this space more and maintained that it was a confined space, although they noticed small connecting channels to Cloquet’s canal in some instances.4
We have utilized spectral-domain OCT5 and swept-source OCT6 to elucidate the anatomy of the posterior vitreous and resolve this decades-old controversy. SS-OCT with an Atlantis DRI-OCT (Topcon) allowed us to compose 18 x 18 mm maps of the posterior vitreous covering an approximately circular area surrounding fixation reaching about halfway to the equator. This coverage exceeded the reach of previous studies, and demonstrated that the bursa extended superiorly beyond the range of our instrument (See Figure 1). This discards the notion of a confined, boat-shaped space and suggests that the bursa may indeed connect to more anterior spaces, as had been proposed originally by Prof. Worst. However, when we examined potential connections of the bursa to other spaces, we found that it fuses superiorly with Cloquet’s canal at a variable distance from the optic nerve. Of note, these were found universally, and also in young eyes without detectable vitreous degeneration. This is quite different from the proposed small connecting channel of the two spaces (Kishi), and a proposed connection of the bursa to a retrociliary circle of cisterns (Worst) and modifies our view of these spaces in a fundamental way: One can imagine these spaces like a mitten, with the thumb part at the beginning of Cloquet’s canal over the optic nerve, the finger part over the macula, and the wrist and arm making their way towards the front of the eye, terminating behind the lens in the space of Erggelet (See Figure 2).
The overall shape of the bursa appears to be quite consistent early in life, but there are significant differences in size (See Figures 3A and B). These may possibly relate to refractive error, according to Prof. Kishi, but we were unable to confirm this in our sample. However, we did observe a “giant premacular bursa” in a father and son with Stickler’s disease and high axial length (See Figure 3C).7
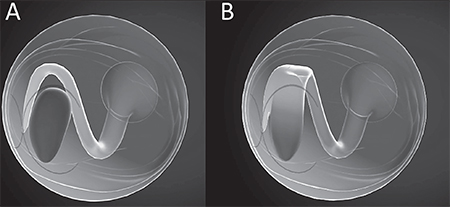 | Figure 2. Juxtaposition of Shoji Kishi’s model of the premacular bursa (A) and our model based on results from SS-OCT mapping of the posterior vitreous (B). Dr. Kishi maintains that the premacular bursa, or “posterior precortical vitreous pocket” is initially a confined, boat-shaped space, which does not connect to any other vitreous spaces except for a small connecting channel to Cloquet’s canal, or connections formed later in life in the context of vitreous degeneration. We propose that the broad fusion of superior extension of the premacular bursa with Cloquet’s canal is already present in young individuals without significant vitreous degeneration, giving rise to a mitten-shaped space, with the thumb connected to the optic nerve and the hand overlying and tethered to the premacular bursa. |
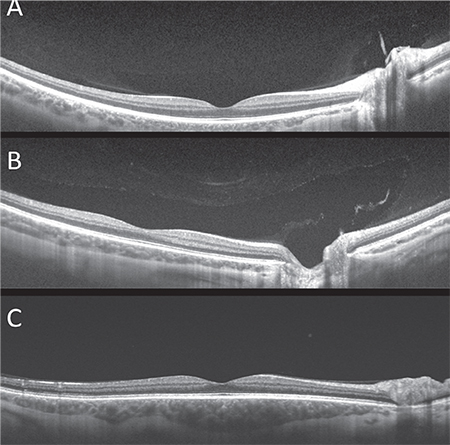 | Figure 3. While the overall shape of the bursa appears to be quite consistent, there may be siginificant differences in size, possibly related to refractive error, age and vitreous degeneration (A, B). In this patient with Stickler’s disease the bursa extends in all dimensions beyond the scan area of 18 x 18 mm (C). |
While examining vitreous scans obtained with SS-OCT, we were struck by some other thin hyporeflective spaces in the vitreous overlying first- and second-order blood vessels in young individuals without vitreous degeneration.8 These probably correspond to the prevascular vitreous fissures observed by Georg Eisner in cadaver eyes with a modified slit lamp.9 In eyes with increasing degrees of syneresis, these spaces appear to widen. In some eyes we found larger, roundish spaces, which likely correspond to the circle of cisterns surrounding the premacular bursa that Prof. Worst had demonstrated with ink injections in cadaver eyes and which can be observed during vitreous surgery employing triamcinolone staining.10 Interestingly, prevascular vitreous fissures were absent in those eyes on our SS-OCT scans. We hypothesize that prevascular vitreous fissures, which were uniformly present in young eyes without vitreous degeneration, enlarge overtime, and are precursors to the cisterns found in cadaver eyes of older individuals with more advanced vitreous degeneration (See Figure 4).
Clinical Utility
Recognition of the normal vitreous anatomy and its characteristic aging changes is helpful in the clinical setting. Recognition of liquid spaces, such as the premacular bursa or vitreous fissures and cisterns may aid in differentiating whether the vitreous is still completely attached, or has completely separated in cases where ultrasound is equivocal. In the above-mentioned case of Stickler’s disease, casual examination of the vitreous scans had suggested an early partial vitreous separation over the temporal macula, but identification of several cisterns was evidence that the bursal wall just mimicked the posterior hyaloid.
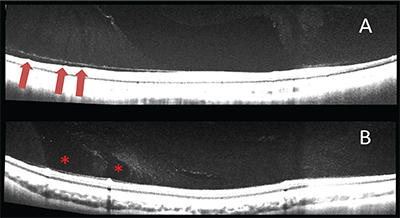 |
| Figure 4. In eyes with little or no detectable vitreous degeneration, hyporeflective thin areas appear to emanate from the larger retinal vessels (red arrows, A). In older subjects with higher degrees of vitreous degeneration, larger areas are found in the same prevascular location (asterisks, B). The former likely correspond to Eisner’s prevascular vitreous fissures, and may represent precursors to the latter, which probably are the perimacular cisterns observed by Prof. Worst. |
The various liquid spaces have been ascribed important roles in a variety of different diseases, including macular hole and epiretinal membrane formation,12 but our understanding of the pathogenesis of these diseases has shifted the focus to the abnormal PVD and cortical vitreous remnants in a phenomenon termed vitreoschisis.
However, they may play an important role in the pathogenesis of proliferative diabetic retinopathy. Prof. Worst and Prof. Kishi both speculated that the “wolf’s jaw” configuration of fibrovascular proliferation in advanced PDR may result from proliferation of neovessels along the bursal wall.12,13 They also hypothesized that the boat-shaped hemorrhages seen in PDR came from bleeding into the premacular bursa. Since PDR can occur in both young and old individuals depending on the onset of diabetes, it is likely that the neovessels grow along different scaffolds, depending on the degree of vitreous separation and the presence or absence of vitreoschisis. One can imagine growth underneath the hyaloid as well as penetration of the latter and proliferation along the walls of the bursa, Cloquet’s canal, prevascular vitreous fissures and cisterns, as well as along vitreoschisis planes. Likewise, hemorrhages may occur in any of those spaces. Preoperative OCT is particularly helpful in these cases, as one can create a virtual map of the various planes and potentially favorable starting points, as well as forming an idea which areas may lend themselves to delamination versus segmentation.
In summary, vitreous imaging with SS-OCT has enhanced our understanding of both the normal vitreous anatomy and its changes during aging, as well as pathological conditions, with potentially important implications for surgical treatment. REVIEW
Dr. Engelbert is an attending physician and research assistant professor at the NYU School of Medicine. Contact him at Vitreous Retina Macula Consultants of New York, P.C., 460 Park Ave., 5th fl. New York, NY 10022. E-mail: michael.engelbert@gmail.com.
1. Worst JGF. The bursa intravitrealis premacularis: New developments in ophthalmology. Doc Ophth Proc Ser 1976:275-9.
2. Kishi S, Shimizu K. Posterior precortical vitreous pocket. Arch Ophthalmol 1990;108:979-82
3. Worst JGF, Los LI. Cisternal anatomy of the vitreous. Amsterdam – New York: Kugler Publications 1995:24
4. Itakura H, Kishi S, Li D, Akyama H. Observation of posterior vitreous pocket using swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 2013;54:3102-7.
5. Pang CE, Freund KB, Engelbert M. Enhanced vitreous imaging technique with spectral-domain optical coherence tomography for evaluation of posterior vitreous detachment. JAMA Ophthalmol 2014;132(9):1148-50.
6. Schaal K, Pang C, Pozzoni AC, Engelbert M. The premacular bursa’s shape revealed by swept-source optical coherence tomography. Ophthalmology 2014;121:1020-8.
7. Chen KC, Jung JJ, Engelbert M. Giant premacular bursa: A novel finding of the posterior vitreous in two patients with Stickler syndrome type 1 revealed by swept-source optical coherence tomography. Graefe’s Arch Clin Exp Ophtahlmol 2015 Aug 6 [Epub ahead of print]
8. Pang CE, Schaal KB, Engelbert M. Association of prevascular vitreous fissures and cisterns with vitreous degeneration as assessed by swept source optical coherence tomography. Retina 2015;35:1875-82.
9. Eisner G. Clinical anatomy of the vitreous. In: Duane TD, Jaeger EA. Biomedical Foundations of Ophthalmology. vol.1, ch.16. Philadelphia: J.B. Lippincott Co., 1990.
10. Fine HF, Spaide RF. Visualization of the posterior precortical vitreous pocket in vivo with triamcinolone. Arch Ophthalmol 2006;124:1663.
11. Chen KC, Jung JJ, Engelbert M. Swept source optical coherence tomography of the posterior vitreous after pars plana vitrectomy. Graefe’s Arch Clin Exp Ophthalmol 22015 Apr 24 [Epub ahead of print]
12. Kishi S. Diagnostic pearls in the management of vitreomacular disorders. Sem Ophthalmol 1998;13:2-9.
13. Worst J. Extracapsular surgery in lens implantation (Binkhorst lecture). Part iv. Some anatomical and pathophysiological implications. J Am Intraocul Implant Soc 1978;4:7-14.



