 A recently approved telescope pros-thesis offers, for the first time, substantial vision and quality-of-life benefits for patients suffering from end-stage age-related macular degeneration. The prosthesis is the third-generation implant (Implantable Miniature Tele-scope by Isaac Lipshitz, MD) from VisionCare Ophthalmic Technologies (See Figures 1 & 2). Wide-angle micro-optics, in combination with the optics of the cornea, create a telephoto system that magnifies objects approximately 2.2 to 2.7 times their normal size. The magnification allows central images to be projected onto healthy perimacular areas of the retina, bypassing, or at least working around, the central scotoma caused by the end-stage AMD patient's foveal geographic atrophy or disciform scar (See Figure 3).
A recently approved telescope pros-thesis offers, for the first time, substantial vision and quality-of-life benefits for patients suffering from end-stage age-related macular degeneration. The prosthesis is the third-generation implant (Implantable Miniature Tele-scope by Isaac Lipshitz, MD) from VisionCare Ophthalmic Technologies (See Figures 1 & 2). Wide-angle micro-optics, in combination with the optics of the cornea, create a telephoto system that magnifies objects approximately 2.2 to 2.7 times their normal size. The magnification allows central images to be projected onto healthy perimacular areas of the retina, bypassing, or at least working around, the central scotoma caused by the end-stage AMD patient's foveal geographic atrophy or disciform scar (See Figure 3).
The telescope prosthesis is approved to improve vision in patients with stable, severe to profound vision impairment (best-corrected distance visual acuity 20/160 to 20/800) caused by bilateral central scotomas associated with end-stage AMD. It is indicated for monocular implantation in the capsular bag (after cataract extraction) for patients 75 years and older.
The telescope-implanted eye provides central vision for improved near and distance functioning with spectacle correction, while the non-implanted fellow eye's peripheral vision allows orientation and safe mobility.
There is a large and growing need for treatments for the end-stage AMD population. Although the past few years have seen huge advances in AMD treatment, in the form of anti-VEGF therapy, a cure remains elusive. Treatments for exudative AMD are not consistently effective1 and there is no treatment for geographic atrophy. Despite our attempts to slow progression of this disease, it often culminates in significant visual acuity loss.
 Currently, about 2 million Americans have advanced AMD, with another 7.3 million at substantial risk of vision loss due to AMD. By 2050, those numbers will likely double, despite current treatments, due to treatment efficacy limitations and demographic trends.2 The consequences for patients are significant. Studies have shown that severe AMD is associated with a 63-percent reduction in quality of life, equivalent to becoming bedridden after a stroke.3 Currently, we have little to offer end-stage patients. Low-vision rehabilitation devices, while beneficial, have not been demonstrated to have a robust impact on quality of life.4
Currently, about 2 million Americans have advanced AMD, with another 7.3 million at substantial risk of vision loss due to AMD. By 2050, those numbers will likely double, despite current treatments, due to treatment efficacy limitations and demographic trends.2 The consequences for patients are significant. Studies have shown that severe AMD is associated with a 63-percent reduction in quality of life, equivalent to becoming bedridden after a stroke.3 Currently, we have little to offer end-stage patients. Low-vision rehabilitation devices, while beneficial, have not been demonstrated to have a robust impact on quality of life.4
Compared to external vision aids, the telescope implant offers a wider field of vision (20-degree to 24-degree field), normal vestibular reflex and vision with natural eye movements in both stationary and dynamic environments. Patients implanted with the device report less difficulty across both task-oriented and psychosocial domains on the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25), such as watching television, recognizing friends and family, seeing facial expressions, reading large print and following sports and theatrical performances.

Two-Year Efficacy Results
A two-year, prospective, multicenter clinical trial was conducted to evaluate the safety and efficacy of the telescope prosthesis. All subjects were ≥55 years old, with severe bilateral impairment due to geographic atrophy or disciform scar, and evidence of cataract. Exclusion criteria included active choroidal neovascularization or treatment for CNV within the previous six months, retinal detachment or retinal vascular disease, previous intraocular or corneal surgery, endothelial cell density (ECD) <1,600 cells/mm2, anterior chamber depth ≤2.5 mm, and myopia >6 D or hyperopia >4 D.
A total of 217 subjects were enrolled at 28 centers. Of those, 206 eyes were implanted with the telescope prosthesis and followed for two years. A subset of 129 subjects continued in the long-term monitoring trial, from which four-year safety data is available. Subjects were required to participate in six low-vision training sessions following implantation of the device. The mean age of subjects enrolled was 76 years, with mean preoperative BCVA of 20/312.
At 12 months, 90.1 percent of subjects had achieved at least a two-line improvement in either near or distance BCVA in the implanted eye, well above the efficacy endpoint of 50 percent established in advance of the trial. Two-thirds (67 percent) of implanted eyes achieved a three-line or greater improvement in distance BCVA versus 13 percent of fellow-eye controls (p=0.0001).5
BCVA improvements were maintained through two years of follow-up.6 At 24 months, 60 percent of the implanted eyes had gained three lines or more, compared to only 10 percent of the fellow eye controls (p<0.00016, See Figure 4). By model, the 3X (nominally 2.7X) device provided mean BCVA improvement of 3.6 lines at two years, versus 2.8 lines for the 2.2X device.
 Visual acuity gains were independent of cataract removal. In 22 fellow eyes that underwent cataract surgery and IOL implantation during the study, and in nine eyes that had IOLs implanted instead of the telescope prosthesis due to intraoperative complications, the mean improvement in acuity was only two letters.6
Visual acuity gains were independent of cataract removal. In 22 fellow eyes that underwent cataract surgery and IOL implantation during the study, and in nine eyes that had IOLs implanted instead of the telescope prosthesis due to intraoperative complications, the mean improvement in acuity was only two letters.6
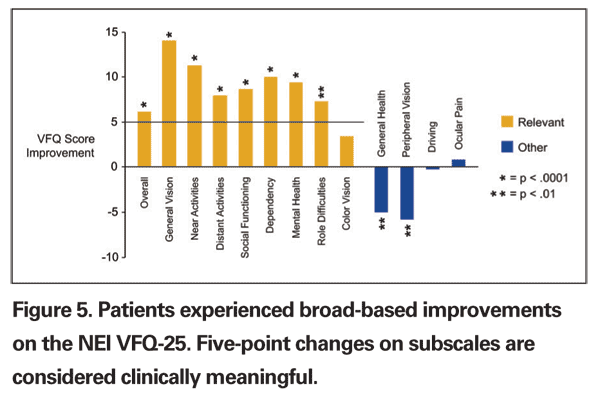
There were also clinically significant increases in quality of life, as measured by the VFQ-25 (See Figure 5). These results, along with our personal clinical experience, suggest that patients are less dependent on others, less worried or frustrated with their visual acuity, less limited in their activities and more able to engage in social interaction with the telescope prosthesis.
Safety, Postop Considerations
Patient follow-up continued for as long as five years, demonstrating a record of long-term safety. The most frequent complications, inflammatory or pigment deposits, are not a significant safety concern. There have been no retinal detachments or cases of endophthalmitis.
In the clinical trial, loss of three or more lines of BCVA occurred in one telescope-implanted eye (0.6 percent) compared to 13 fellow-eye controls (7.5 percent) at two years.6
The most significant risk of the procedure is to corneal health. Endothelial cell loss at 12 months was higher than anticipated, with a 25-percent reduction from baseline. However, the rate of ECD loss between one and two years was just 2.4 percent.6 The pattern of ECD loss appears to be similar to that of routine cataract surgery, but with a larger initial loss due to the surgical insult, followed by a much lower rate of chronic ECD loss. Overall, the rate of corneal transplant over five years of follow-up was 2.3 percent, or five eyes, of which three successfully retained the telescope.
Explantation of the telescope prosthesis occurred for various reasons. Two were explanted early in the study as a result of device failures, two during corneal transplantation, and eight at the request of the patient because of dissatisfaction. There was a higher rate of dissatisfaction among patients with hereditary Stargardt's disease, likely due to long-standing compensatory visual strategies. The procedure is contraindicated in this patient population.
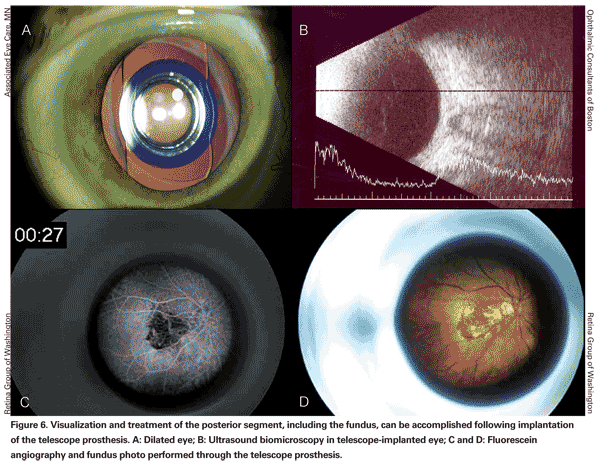
Visualization and treatment of the posterior segment, including the fundus, can be accomplished following implantation of the telescope prosthesis (See Figure 6). Fundus visualization can be performed at the slit lamp through a 90-D lens. Peripheral visualization can be performed by indirect ophthalmoscopy with the eye fully dilated, so that the examiner can observe the retina around the telescope prosthesis.
A case of successful thermal laser photocoagulation of a choroidal neovascular lesion through the telescope device has been reported in the literature.7 The authors noted that fluorescein angiographic imaging was more challenging than in typical eyes, but that they were able to clearly see the necessary landmarks to safely perform the laser treatment.
Although a rare occurrence in the long-term follow-up (one case), visually significant posterior capsule opacification can be resolved with a needling procedure in patients with the telescope prosthesis. A pars plana approach, using a 25-ga. vitrector to engage the posterior capsule and perform a central posterior capsulotomy, has also been described.8
CentraSight Treatment Program
The telescope prosthesis is part of a comprehensive treatment program. By utilizing the skill sets of a multidisciplinary team, the CentraSight program helps to maximize the utility of the device for visually impaired patients.
The treatment program involves four steps: 1) diagnosis of end-stage AMD by a retina specialist; 2) candidate screening, using external telescope simulators, by low-vision professionals working with the retina specialist; 3) implantation of the telescope by a cornea-trained cataract surgeon; and 4) postoperative visual rehabilitation with a low-vision specialist and occupational therapist.
Retina specialists serve as the conduit for these patients: Our role in identifying, diagnosing and screening candidates is critical. Among our patient populations are many individuals who have failed treatment with anti-VEGF and other, older therapies. Provided their neovascular disease is stable, they may be good candidates for telescope implantation. Many others, especially our atrophic AMD patients, are no longer under care because of the dearth of treatment options, but the availability of this device and other clinical trials under way may bring some of them back in for evaluation.
After determining that the patient meets the requirements for the telescope prosthesis, and that no contraindications are present, we can refer them on to other members of the treatment team to handle the initial evaluation and care through implantation and early postoperative follow-up, after which they are likely to return to us for long-term management.
Low-vision professionals help the team in areas not part of the retina or cornea specialists' practice patterns. They manage patients' expectations and determine their willingness and ability to participate in postoperative visual training. They also play an important role in helping us and our cornea colleagues select the best eye for implantation, and to determine the degree to which the implanted device will benefit the patient, by using an external telescope simulator (ETS). Their participation in the course of care is required as part of the approved labeling, and is a critical component in successful implantation and rehabilitation.
In clinical trials, the telescope was implanted in most cases in the eye with poorer visual acuity. However, implantation in the better-seeing or dominant eye may enhance success rates with the device.9 A systematic protocol for determining the best eye based on responses to the ETS has been developed and used in the
Although Food and Drug Administration-approved, Medicare coverage must be finalized before most patients will have access to the procedure. Implantation of the telescope prosthesis will initially be an outpatient, hospital-based procedure, per Medicare's new-technology device coding regulations. Medicare coverage and payment codes are expected to become available in the first half of 2011.
Initially, retina specialists who are not already leading a CentraSight team can help their end-stage AMD patients by referring them to a regional team. After Medicare coverage is obtained, additional multispecialty practice and referral network teams will be trained in order to expand access to all patients.
Using human value metrics typically applied in the
As for the future of our patients with significant visual loss due to AMD, there are a number of other promising devices and therapies under investigation for visually impaired patients, including gene therapy (for which there is a Phase I wet AMD trial under way), stem cell transplantation and retinal prosthetic devices. Most of these have been initially targeted towards overall retinal degenerative diseases such as Leber's congenital amaurosis and retinitis pigmentosa, and may be less suited to end-stage AMD. In any case, as these other alternatives are years away from approval and commercial availability, the newest weapon for the foreseeable future in our end-stage AMD visual rehabilitation armamentarium is the telescope prosthesis.
The telescope prosthesis is an FDA-approved treatment for patients with previously very limited treatment options. This treatment is delivered through a comprehensive, multidisciplinary approach that restores some central vision to appropriately selected patients, for meaningful vision and quality-of-life gains, and represents an exciting and important step forward in the care of our patients with end-stage AMD.
Dr. Haller is Ophthalmologist-in-Chief of the Wills Eye Institute and professor and chair of the department of ophthalmology at
Dr. Heier is is a vitreoretinal specialist at Ophthalmic Consultants of Boston and co-director of the Vitreoretinal Fellowship at OCB/Tufts University Medical School. Contact him at
jsheier@eyeboston.com.
2. Rein DB, Wittenborn JS, Zhang X, et al, and Vision Health Cost-Effectiveness Study Group. Forecasting age-related macular degeneration through the year 2050: The potential impact of new treatments. Arch Ophthalmol 2009;127:533-40.
3. Brown MM, Brown GC, Sharma S, et al. The burden of age-related macular degeneration: A value-based analysis. Curr Opin Ophthalmol 2006;17:257-66.
4. Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: A systematic review. Can J Ophthalmol 2008;43:180-7.
5. Hudson HL, Lane SS, Heier JS, et al. Implantable miniature telescope for the treatment of visual acuity loss due to end-stage age-related macular degeneration: One-year results. Ophthalmology 2006;113:1987-2001.
6.
7. Garfinkel RA, Berinstein DM, Frantz R. Treatment of choroidal neovascularization through the implantable miniature telescope. Am J Ophthalmol 2006;141:766-7.
8. Singer MA, del Cid MR, Stelton CR, Boord T. Pars-plana posterior capsulotomy in a patient with a telescope prosthesis for age-related macular degeneration. Arch Ophthalmol 2010;128:1065-7.
9. Primo SA. Implantable miniature telescope: Lessons learned. Optometry 2010 ;81:86-93.



