Mark B. Abelson, MD, CM, FRCSC, and Aron Shapiro
Since they were first recognized by Wallace P. Rowe and colleagues in 1953,1 seven species and 54 serotypes of adenovirus have been identified.2-4 They are known to cause upper and lower respiratory tract infections, several varieties of viral conjunctivitis (including keratoconjunctivitis), as well as gastroenteritis and hemorrhagic cysts, though only certain serotypes are associated with each. With regard to ocular infections, adenoviruses present a serious public health risk and are responsible for 65 to 90 percent 5,6 of viral conjunctivitis and 15 to 70 percent of all cases of infectious conjunctivitis worldwide,5 with a majority of outbreaks occurring in
There are no known cures for adenoviral infections and therapeutic options are limited to alleviating symptoms and allowing the virus to subside naturally. Unfortunately, during this period, which can last up to two weeks, the patient remains infectious and capable of transmitting the disease to others. In this article, we'll discuss the serotypes related to ocular manifestations of viral infection and the consequences of adenoviral outbreaks, as well as the future of prevention and treatment.
Ocular Manifestations
Adenoviruses are non-enveloped, double-stranded-DNA viruses with icosahedral capsids.9 Of the seven species (A to G), species A has been associated with the gastrointestinal tract,10 while species B and C are more common to the respiratory tract,10 though conjunctivitis outbreaks have been associated with adenovirus type 3 (species B). Species D overwhelmingly causes conjunctivitis,5,10,11 and species E is found in respiratory and ocular infections, but more commonly in conjunctivitis.10 Finally, species F, and the more recently discovered species G, are the agents of gastroenteritis.10
Distinguishing between adenoviral and bacterial conjunctivitis can be achieved via laboratory assay or through differential diagnosis of signs and symptoms. Acute adenoviral conjunctivitis is more common in individuals over 12 years old, while bacterial acute conjunctivitis is more common in children under 12.12 A burning sensation, an enlarged and painful preauricular lymph node, corneal involvement and a watery discharge are typically more prominent in adenoviral cases than in bacterial cases of acute conjunctivitis, which are typified by mucopurulent discharge.
It's important to examine the patient for both the presence of preauricular lymphadenopathy and a follicular reaction. Approximately one-third to one-half of adenovirus cases have a preauricular node and associated follicles because both are a part of the same lymphogenesis in which the body is responding to a novel antigenic stimulant. Thus, it's essential to feel the preauricular node and pull down the lid to look for follicular bumps. However, these may not be detectable during the first few days of infection and are nonspecific for adenoviral disease.
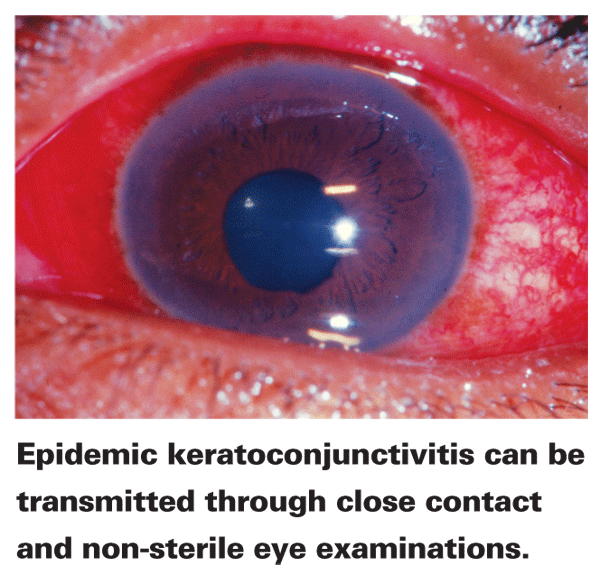
Adenoviral ocular infections are classified into four distinct syndromes: pharyngoconjunctival fever (PCF); epidemic keratoconjunctivitis (EKC); acute nonspecific follicular conjunctivitis (NCF) and chronic keratoconjunctivitis. PCF is most commonly found in children and is usually caused by adenovirus serotype 3, but has also been associated with types 1, 4, 5, 6, 7 and 14. Following an incubation period of five to 12 days, a patient with PCF will usually present with a fever lasting for about 10 days.
The infection typically occurs bilaterally and is usually accompanied by a sore throat, slight burning, irritation and mild photophobia. Lid swelling may also occur in the first few days of infection and, about a week after symptom onset, punctate keratitis may develop, followed by corneal infiltrates. Although PCF infection typically self-resolves within a few weeks, the disease is highly communicable.13
EKC is the most serious form of ocular adenoviral infection and is usually associated with types 8 and 19. It has also been reported with adenivirus types 2 to 4, 7 to 11, 14, 16 and 29. EKC can be transmitted via close personal contact as well as during non-sterile eye examinations with contaminated ophthalmic instruments.14 Close-quarter environments, such as hospitals, doctors' offices, industrial areas and swimming pools are prime environments for the spread of the disease.13 After an eight-day incubation period, tears and saliva are contagious for about two weeks; conjunctivitis almost always resolves in two to three weeks.13
EKC is most common during the fall and winter and, in contrast to PCF, presents unilaterally in two-thirds of cases and doesn't cause fever or sore throat. Keratitis occurs in ap--proximately 80 percent of patients with associated discomfort, photophobia, tearing and mild blepharospasm. Following redness and keratitis, up to 20 uniform, subepithelial corneal infiltrates (the hallmark of EKC) develop on day 11 and are most prevalent during the third and fourth weeks of infection. Approximately 30 to 50 percent of patients with EKC will de-velop these infiltrates, which may contribute to persistent visual loss and light sensitivity and necessitate long-term steroid therapy. The infiltrates are a product of the immune response to the keratitis and are smaller, more numerous, denser, produce greater photophobia and last longer (up to a year) than PCF infiltrates.
In addition, EKC may lead to persistent dry eye or conjunctival scarring.1
Acute nonspecific follicular conjunctivitis may be caused by many of the viral serotypes that induce PCF or EKC, but it doesn't involve the cornea and the resulting conjunctivitis is typically mild. Nonspecific follicular conjunctivitis usually resolves itself within a week to 10 days but can serve as a reservoir of infectious adenovirus which may lead to a widespread epidemic.13
The fourth and by far the rarest form of ocular adenoviral infection is chronic keratoconjunctivitis. It's characterized by intermittent periods of tearing, redness and photophobia, which can last for up to 18 months. Patients will almost always have had a recent episode of acute conjunctivitis several months preceding onset.13
Transmission and Outbreak
Adenoviruses are robust, resilient to standard disinfection and can easily be transmitted in settings with a high population density or a heavy flow of people. They are spread through droplets from the respiratory tract and eye, or through such secretions on an intermediate vehicle (e.g., a contaminated medical device or unclean surface) through a process known as viral shedding.15 Viral shedding refers to the method by which a virus present on the body is exuded out to the environment or onto another body part. Once adenoviruses successfully replicate within a host cell and have exhausted all host-cell resources in making their progeny, the virus induces cell lysis and begins its search for new, viable hosts. This can mean infection of other tissues as well as shedding onto environmental surfaces.
Environmental contamination with adenovirus is a common source of infection, and eye clinics are especially fertile grounds for outbreaks. For instance, one Canadian clinic experienced a significant EKC outbreak in 1995 before infection control measures were fully adopted. In this case, implementation of seemingly simple measures such as hand washing, instrument cleaning with buffered bleach solution, triaging suspected cases to separate waiting areas and the use of gloves halted the outbreak.16
Unapparent ocular infections during an epidemic may also contaminate surfaces and infect others through asymptomatic shedding.17 In a recent Japanese study, investigators performed a single conjunctival swab of 17 asymptomatic ophthalmology ward inpatients and an asymptomatic ophthalmologist to test for the presence of adenovirus after one inpatient was diagnosed with serotype 37 adenoviral conjunctivitis.
Despite the lack of symptoms, which continued from the time of hospitalization until eye surgery three weeks later, adenovirus antigen was detected in one patient and adenovirus DNA was present in eight others. These results suggest that mild or unapparent infection is possible during outbreaks and may play a role in transmission. In this case, the possible routes of transmission were narrowed down to the room and instruments used for eye exams and operations, which were common among all 10 infected persons, but the exact route remained undetermined.17 The study also uncovered no evidence of adenovirus DNA with PCR analysis in 30 asymptomatic control cases not hospitalized during the outbreak, reinforcing the belief that Ad is not part of the normal conjunctival flora.17 This finding is consistent with a previous study in which all 102 controls tested negative (using cell culture with direct immunofluorescence) for the presence of adenovirus in normal flora.7
Contact lens wearers with adenoviral infections should dispose of their lenses once their illness has resolved, as studies have found both serotypes 8 and 19 can survive both heat and hydrogen peroxide sterilization systems. Contaminated lenses may serve as a reservoir for the spread of infection to others and the virus can survive both chemical and hydrogen peroxide sterilization systems.18
Detection
There are several techniques for the detection of adenovirus, either in the laboratory or at the point of care. Cell culture with confirmatory immunofluorescence assay (CC-IFA) allows for visualization of adenovirus proteins or antibodies through binding to fluorescent dye. For the test to be positive, the viral particles must be capable of entering a cell, replicating and producing infectious prodigy virions. The visualization method is indirect, where a secondary antibody labeled with fluorochrome is used to recognize a primary antibody. CC-IFA is also used in detecting herpes and influenza (including H1N1) viruses. A positive CC-IFA cell culture shows that a patient is harboring live virus and is infective. As a result, the assay has historically been considered the gold standard of adenovirus detection.
PCR replicates viral genetic material, detecting not only live virus but also incomplete or dead viral material. This method is therefore a better measure of the total quantity of virus present in a given sample, with a low amount of amplified adenoviral DNA suggesting an inactive or resolved infection. Aside from CC-IFA and PCR, laboratory antigen tests, immunochromatography and enzyme im--munoassays may also be used.
The first point-of-care test approved by the U.S. Food and Drug Administration is the rapid pathogen screening Adeno Detector (Rapid Pathogen Screening,
To perform the test, a sterile strip is used to collect tears from the palpebral conjunctiva (with or without the use of a local anesthetic) and is then dipped into the buffer solution. Antigens bind to monoclonal antibodies on the test strip and subsequently leave a one-line (negative) or two-line (positive) mark. The test can detect all known adenovirus serotypes.12
Treatment and other Considerations
Since viruses incorporate themselves within host cells and use host cell machinery for replication, they are notoriously hard to treat without unwanted toxic effects.
Adenoviruses are no exception as there are currently no available treatments for these diseases, although most infections typically resolve themselves. In severe cases, anti-inflammatory and anti-immune therapies are desirable for reducing irritation, visual disturbances, photophobia, pain, lid swelling, chemosis and psuedomembranes and for preventing the formation of subepithelial immune corneal infiltrates.20 A topical steroid can be prescribed to relieve symptoms, though the use of steroids for the treatment of adenoviral conjunctivitis remains controversial. Steroids mask the symptoms of the disease without having an effect on the underlying virus, and there is evidence that steroids may prolong the contagion period by promoting replication and prolonging shedding.20
Novel treatment avenues have been pursued but none has been approved, such as the development of an ophthalmic formulation of the antiviral cidofovir, which was unfortunately halted during human clinical trials due to ocular toxicity. Likewise, an adenoviral vaccine was developed and used by the
A povidone-iodine 0.4% and dexamethasone 0.1% ophthalmic suspension (Foresight Biotherapeutics) is currently in development for the treatment of adenoviral conjunctivitis. Povidone-iodine (PVP-I), which works by iodination of lipids and oxidation of cytoplasmic and membrane compounds, may provide antimicrobial activity with no known risk of microbial resistance. In a study of an animal model of adenoviral conjunctivitis presented by
In a 2009 pilot study by Jesse Pelletier and colleagues at the Ocean Ophthalmology Group in North Miami Beach, Fla., that used positive results from the RPS Adeno Detector along with PCR and CC–IFA as inclusion criteria, PVP-I/dexamethasone showed efficacy in reducing both the inflammatory and infectious components of the disease.22 Clinical resolution was achieved by day three or four in eight of nine study eyes and elimination of infectivity (measured by CC-IFA) was accomplished by day four or five in five of six eyes with in--fectious adenovirus at enrollment.22
Phase II trials have begun for a novel compound called NVC-422 (Aganocide, NovaBay/Alcon) for the treatment of viral conjunctivitis. The drug is composed of proprietary analogs to the N-chlorinated molecules used by white blood cells to destroy viruses and bacteria.23 Additionally, a drug known as EkcCide (Nanoviricides) is currently in preclinical development and has shown efficacy in eliminating EKC symptoms in rabbits infected with adenovirus 5. EkcCide is part of a new class of drugs specifically designed to attack enveloped virus particles and dismantle them.24
Ocular adenoviral infections represent a serious public health risk due to their rapid spread (especially in the case of EKC) and propensity for severe symptoms. The disease is most easily missed or confused with other conditions at the earliest stages when the physician may contaminate himself and spread the disease to others, so a high level of vigilance and hygiene is essential. There is currently no FDA-approved medicine for adenoviral conjunctivitis, though we hope that new therapeutics currently being researched will meet this need. In the meantime, proper identification of adenoviral infections will allow the ophthalmologist to manage the disease and to advise the patient to avoid situations where he or she could infect others.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Rowe WP, Huebner RJ, Gilmore LK, et al. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 1953;84:3:570-3.
2. Benko M. Comparison of the genome of ovine adenovirus types 1 through 5 by restriction enzyme analysis and DNA hybridisation. Acta Vet Hung 2000;48:4:477-84.
3. Jones MS, 2nd, Harrach B,
4. Ishiko H, Shimada Y, Konno T, et al. Novel human adenovirus causing nosocomial epidemic keratoconjunctivitis. J Clin Microbiol 2008;46:6:2002-8.
5. Maranhao AG, Soares CC, Albuquerque MC, Santos N. Molecular epidemiology of adenovirus conjunctivitis in Rio de Janeiro, Brazil, between 2004 and 2007. Rev Inst Med Trop Sao Paulo 2009;51:4:227-9.
6. Abelson MB, Allansmith MR. Normal conjunctival wound edge flora of patients undergoing uncomplicated cataract extraction. Am J Ophthalmol 1973;76:4:561-5.
7. Gigliotti F, Williams WT, Hayden FG, et al. Etiology of acute conjunctivitis in children. J Pediatr 1981;98:4:531-6.
8. Kaneko H, Ishiko H, Ohguchi T, et al. Nucleotide sequence variation in the hexon gene of human adenovirus type 8 and 37 strains from epidemic keratoconjunctivitis patients in
9. Swenson PD, Wadell G, Allard A, Hierholzer JC. Adenoviruses. In: Manual of Clinical Microbiology, 8 ed, vol 2.
10. Chang SY, Lee CN, Lin PH, et al. A community-derived outbreak of adenovirus type 3 in children in
11. Sambursky RP, Fram N, Cohen EJ. The prevalence of adenoviral conjunctivitis at the Wills Eye Hospital Emergency Room. Optometry 2007;78:5:236-9.
12. O'Brien TP, Jeng BH, McDonald M, Raizman MB. Acute conjunctivitis: Truth and misconceptions. Curr Med Res Opin 2009;25:8:1953-61.
13. Pavan-Langston D. Viral Diseases of the Cornea and External Eye. In: Power WJ, Azar DT, eds. Principles and Practices of Ophthalmology, vol 1.
14. Tullo AB. Shipyard eye. Br Med J (Clin Res Ed) 1981;283:6298:1056-7.
15. Langley JM. Adenoviruses. Pediatr Rev 2005;26:7:244-9.
16. Montessori V, Scharf S, Holland S, et al. Epidemic keratoconjunctivitis outbreak at a tertiary referral eye care clinic. Am J Infect Control 1998;26:4:399-405.
17. Kaneko H, Maruko I, Iida T, et al. The possibility of human adenovirus detection from the conjunctiva in asymptomatic cases during nosocomial infection. Cornea 2008;27:5:527-30.
18. Kowalski RP, Sundar-Raj CV, Romanowski EG, Gordon YJ. The disinfection of contact lenses contaminated with adenovirus. Am J Ophthalmol 2001;132:5:777-9.
19. Sambursky R, Tauber S, Schirra F, et al. The RPS adeno detector for diagnosing adenoviral conjunctivitis. Ophthalmology 2006;113:10:1758-64.
20. Romanowski EG, Pless P, Yates KA, Gordon YJ. Topical cyclosporine A inhibits subepithelial immune infiltrates but also promotes viral shedding in experimental adenovirus models. Cornea 2005;24:1:86-91.
21. Russell KL, Hawksworth AW, Ryan MA, et al. Vaccine-preventable adenoviral respiratory illness in US military recruits, 1999-2004. Vaccine 2006;24:15:2835-42.
22. Pelletier JS, Stewart K, Trattler W, et al. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther 2009;26:8:776-83.
23. Alcon Commences Phase 2 Clinical Trial of NovaBay's NVC-422 for Viral Conjunctivitis. NovaBay website. http://www.novabaypharma.com/investors/release/july_05_2009. Accessed January 22, 2010.
24. Nanoviricides, Inc. http://www.nanoviricides.com. Accessed January 22, 2010.



