|
No new ICD-9 codes were published in anticipation of
ICD-10 implementation on October 1, 2015. |
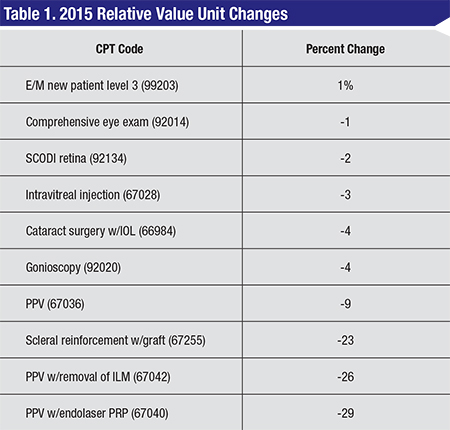
A. There are several changes to the Medicare Physician Fee Schedule, which was published in November 2014. Please see the table, below, for examples of some of the relative value units changing and the percentage of change from 2014.
Q. Will there be more than one fee schedule in 2015?
A. On November 13, 2014, the Federal Register contained the final rule for the physician fee schedule and other Medicare Part B payment policies. Several factors affect payment policies:
• The Protecting Access to Medicare Act 2014 preserves a 0 percent physician fee schedule update for January 1 to March 31, 2015.
• The conversion factor changes slightly from $35.8228 to $35.7547 on January 1. If Congress does not intervene prior to April 1, the conversion factor will drop to $28.2239 for April 1 through December 31, 2015.
• Relative Value Unit changes will occur on January 1, creating a new fee schedule for the first quarter of 2015. In addition, the Centers for Medicare & Medicaid Services corrected an error in the malpractice RVUs, which results in an approximately 1 to 2 percent reimbursement reduction for ophthalmology.
Q. What is happening to ambulatory surgery center facility fees in 2015?
A. For 2015, the wage adjustment for budget neutrality, in addition to the multifactor productivity adjusted update factor, increases the ASC conversion factor by 1.4 percent for those centers that meet quality reporting requirements, resulting in small increases in facility reimbursement. ASCs began reporting National Quality Forum Measures in October 2012. The reporting of these measures also affects reimbursement. Nonparticipation or failure to meet the necessary requirements results in a 2-percent reduction to facility Medicare reimbursement.
 |
A. Yes. Various adjustments to hospital reimbursement result in a Hospital Outpatient Department rate increase of 2.3 percent.
Q. What changes were published with Category I codes in CPT 2015?
A. The CPT 2015 coding manual contains a number of new codes, revisions and deletions applicable to ophthalmology. Category I CPT code changes are as follow:
• 66179 Aqueous shunt to extraocular equatorial plate reservoir, external approach; without graft (new);
• 66180 with graft (revised) (Do not report 66180 in conjunction with 67255);
• 66184 Revision of aqueous shunt to extraocular equatorial plate reservoir; without graft (new);
• 66185 with graft (revised) (Do not report 66185 in conjunction with 67255);
• 92145 Corneal hysteresis determination, by air impulse stimulation, unilateral or bilateral, with interpretation and report (replaces 0181T).
The following code was revised:
• 67399 Unlisted procedure, ocular extraocular muscle.
The following code was deleted:
• 66165 Fistulization of sclera for glaucoma; iridencleisis or iridotasis.
Q. Were there any Category III code changes published in 2015?
A. There were a number of changes. These Category III codes, implemented on July 1, 2014 now appear in the hard copy CPT 2015:
• 0341T Quantitative pupillometry with interpretation and report, unilateral or bilateral;
• 0356T Insertion of drug-eluting implant (including punctal dilation and implant removal when performed) into lacrimal canaliculus, each.
New Category III codes include:
• 0378T Visual field assessment, with concurrent real time data analysis and accessible data storage with patient initiated data transmitted to a remote surveillance center for up to 30 days; review and interpretation with report by a physician or other qualified health care professional;
• 0379T Technical support and patient instructions, surveillance, analysis, and transmission of daily and emergent data reports as prescribed by a physician or other qualified health care professional;
• 0380T Computer-aided animation and analysis of time series retinal images for the monitoring of disease progression, unilateral or bilateral, with interpretation and report.
Revised Category III codes include:
• 0191T Insertion of anterior segment aqueous drainage device, without extraocular reservoir, internal approach, into trabecular meshwork; initial insertion (revised text in bold).
+ 0376T each additional device insertion (List separately in addition to code for primary procedure) (new);
• 0253T Insertion of anterior segment aqueous drainage device, without extraocular reservoir, internal approach, into the suprachoroidal space (revised text in bold).
Coverage and payment for Category III codes remains at carrier discretion.
Q. Are there new drug codes pertinent to ophthalmology in 2015?
A. Yes; one new HCPCS Code that is effective January 1, 2015 is for the proprietary combination of phenylephrine 1.0% combined with ketorolac 0.3% (Omidria), which received pass-through status at the end of October 2014. Omidria is used during cataract and lens-replacement surgery to maintain pupil size by preventing intraoperative miosis and to reduce postoperative pain.
• C9447 Injection, phenylephrine and ketorolac, 4 ml vial.
This is submitted by the ASC or HOPD.
Q. Were there any changes to diagnosis codes or the implementation of ICD-10?
 |
Q. What types of regulatory issues were identified in the annual Office of Inspector General Work Plan as areas of concern for ophthalmology in 2015?
A. The annual publication included a series of initiatives that will continue through 2015. No “new” initiatives appear pertinent to ophthalmology. The returning targets for scrutiny include:
• Place of Service Errors;
• Payments for drugs;
• Ambulatory Surgical Centers–Payment System;
• Ophthalmological Services–Questionable billing during 2012;
• Imaging services–Payments for Practice Expense;
• Medicare Incentive Payments for Adopting Electronic Health Records;
• Anesthesia services–Payments for personally performed services;
• Payment for compounded drugs under Medicare Part B; and
• Security of Certified Electronic Health Record Technology under Meaningful Use.
Q. Are there any changes to the Recovery Audit Contractor Program?
A. Yes. The RAC program “paused” in February 2014 while CMS negotiated new contracts for the RACs. Some automated reviews resumed in August 2014 and changes to the program are expected in 2015. Total corrections since the Medicare Fee-for-Service Recovery Audit Program began in October 2009 stand at $7.26 billion with $6.8 billion in overpayments.
Q. Are there penalties and/or bonus dollars for participation in the Physician Quality Reporting System in 2015?
A. The Patient Protection and Affordable Care Act made PQRS mandatory by 2015. Those eligible professionals who did not successfully report PQRS in 2013 will be paid 1.5 percent less than the MPFS reimbursement rate in 2015. Unsuccessful reporters in 2014 will receive a 2 percent PQRS penalty adjustment (receiving 98 percent of the fee schedule amount) in 2016. Bonus payments ended with the 0.5 percent bonus paid to successful reporters in 2014. No bonus payments exist for 2015.
Q. Is the Electronic Health Record Bonus Program continuing in 2015?
A. As of September 2014, the EHR Incentive Bonus Program paid out $6.47 billion to eligible providers; $187 million was to ophthalmologists and $261 million was to optometrists. Providers expected to attest to Stage 2 requirements for 2014 received a reprieve due to a variety of vendor issues and other hang-ups. Most reported Stage 1 meaningful use objectives and measures for 2014 and will move forward with Stage 2 in 2015. Meaningful use reporting in 2015 requires full-year participation for anyone beyond year one reporting. Providers who did not qualify for a hardship exemption or complete their meaningful use attestation for Stage 1 by October 1, 2014 will receive a 1 percent penalty on their MPFS reimbursements for 2015.
Q. What Medicare Part B changes affect beneficiaries in 2015 from a cost perspective?
A. The Medicare Part B premiums remain $104.90 for most beneficiaries. The Part B deductible also remains at $147.00. These beneficiary costs are unchanged from 2013 and 2014. REVIEW
Ms. McCune is vice president of the Corcoran Consulting Group. Contact her at DMcCune@corcoranccg.com.



